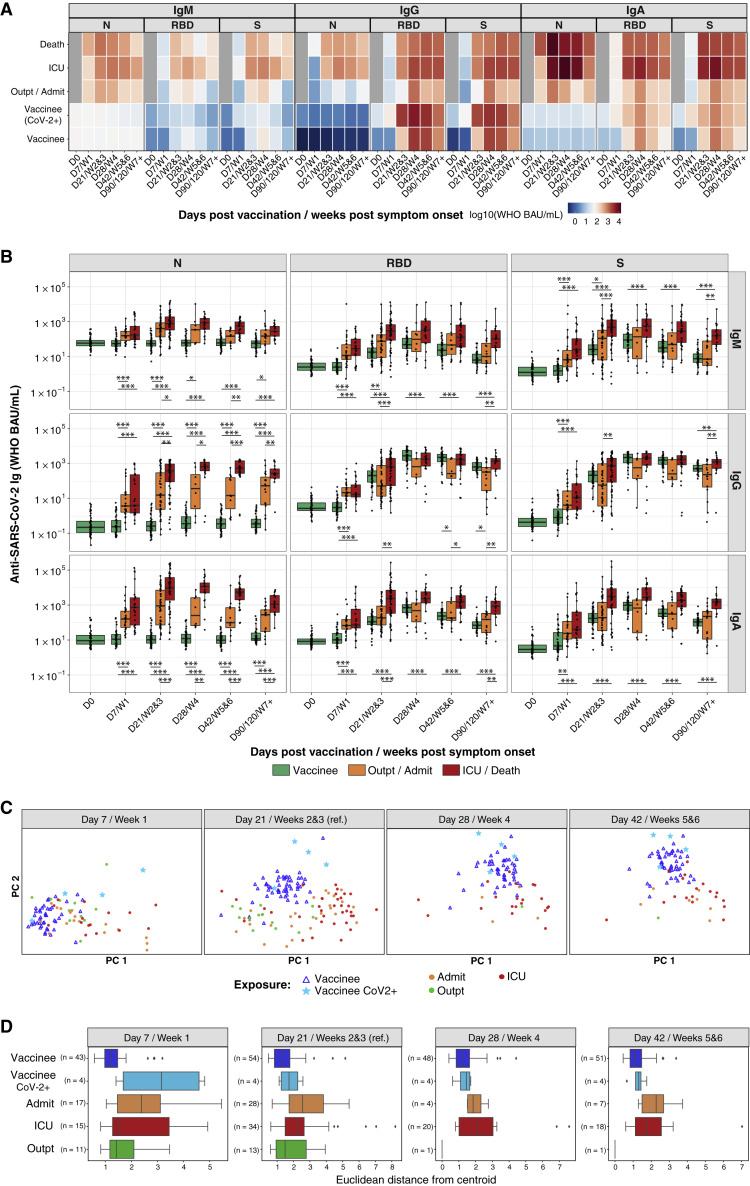
Figure 2.
BNT162b2 vaccination and SARS-CoV-2 infection elicit distinct antibody profiles
(A and B) Anti-SARS-CoV-2 N, RBD, and spike (S) IgM, IgG, and IgA antibody responses are shown for individuals who received BNT162b2 prime (D0) and second (D21) vaccination doses and for COVID-19 patients. (A) The heatmap shows the development of antibody responses in longitudinal samples from vaccinees/patients collected at D0, D7/week 1, D21/weeks 2 and 3, D28/week 4, D42/weeks 5 and 6, and D90/120/≥week 7 after vaccination/COVID-19 symptom onset (x axis). The color scale encodes the median values of log10 WHO BAU/mL Ig concentrations. (B) Box-whisker plots show the development of antibody responses in longitudinal samples from vaccinees/patients collected at D0, D7/week 1, D21/weeks 2 and 3, D28/week 4, D42/weeks 5 and 6, and D90/120/≥week 7 after vaccination/COVID-19 symptom onset (x axis). Box-whisker plots show the interquartile range as the box and the whisker ends as the most extreme values within 1.5 times the interquartile range below the 25% quantile and above the 75% quantile. Statistical test: pairwise Wilcoxon rank sum test with Bonferroni correction. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001. Individuals were classified as outpatients (Outpt) and hospital-admitted patients (Admit); ICU patients and those who died from their illness (Death); and vaccinees who had (CoV-2+) or had not had a positive SARS-CoV-2 test in the past.
(C) PCA of anti-SARS-CoV-2 RBD, N-terminal domain, and S (but not N) IgM, IgG, and IgA concentrations across BNT162b2 vaccinees and Wuhan-Hu-1-infected Stanford COVID-19 patient cohort 1 at different time points after vaccination/COVID-19 symptom onset visualized on a consistent PCA reference created using D21/weeks 2 and 3 as a reference time point.
(D) Distribution of Euclidean distances between BNT162b2 vaccinee samples and their centroid, compared with Wuhan-Hu-1-infected Stanford COVID-19 patient cohort 1 samples and their centroid, at different time points after vaccination/COVID-19 symptom onset.