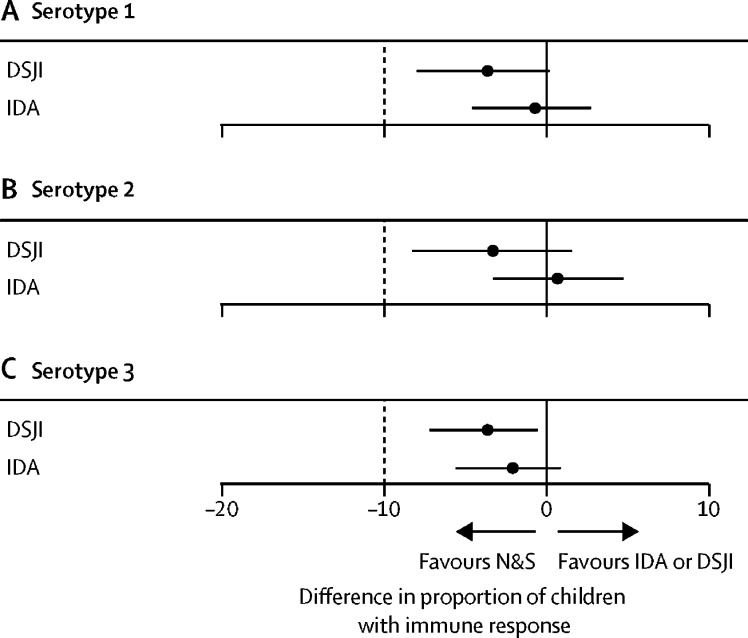
Figure 3.
Effects of administration method on type-specific immune responses
Differences in the percentage of participants having an immune response to poliovirus type 1, poliovirus type 2, or poliovirus type 3 after the administration of an intradermal fractional dose of inactivated poliovirus vaccine via IDA or DSJI, compared with the reference N&S method. The percentage of children who had an immune response after intradermal fractional dose inactivated poliovirus vaccine was calculated combining the percentage who underwent seroconversion (baseline SNA titres of <8 and a post-vaccination titre of ≥8) with the percentage who were seropositive (SNA≥8) at baseline and had a four-fold rise in SNA titres post-vaccination. Children with a baseline titre of >362 were excluded from the analysis because a four-fold rise was beyond the upper limit of quantification the assay (table 3). Point estimates and 97·5% CI are illustrated. The 97·5% CI were adjusted for age, sex, and variables associated with the immune response in a multivariable regression model developed for each type (type 1: baseline seropositivity, appendix p 8; type 2: number of previous oral poliovirus vaccine doses, time taken to vaccinate, maternal occupation, appendix p 9; type 3: baseline seropositivity, appendix p 10). DSJI=disposable syringe jet injector. IDA=intradermal adapter. N&S=needle and syringe. SNA=serum neutralising antibody.