FIGURE 1.
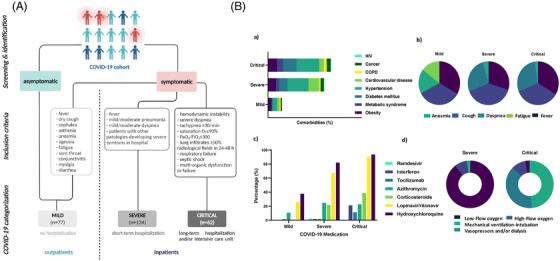
Study design and clinical characterization of the study cohort. (A) Flowchart of the clinical strategy followed to categorize patients of the coronavirus disease‐2019 (COVID‐19) study cohort. (B) Incidence of comorbidities (a), COVID‐19 symptoms (b), medication (c), and oxygen & intensive care (d), grouped by disease severity as mild, severe and critical patients. The size of bars (a, c) and circular (b, d) portions is proportional to the percentage of the corresponding comorbidity, symptom, medication or treatment. While patients with mild disease presented mostly anosmia, were treated with antibiotics and did not require oxygen supply, the incidence of dyspnea was significantly higher in the severe and critical groups, many of the latter requiring corticosteroids, hydroxychloroquine, lopinavir/ritonavir. Low‐flow oxygen therapies were mainly necessary for severe patients, some of who required high‐flow oxygen administration and, a high proportion of critical patients were intubated and required vasopressor administration or dialysis. Please note that COVID‐19‐related medication (b) was dispensed after blood sample collection, so that is assumed the subsequent analysis are not biased due to exposure to medication at the time of blood collection
