Figure 22.
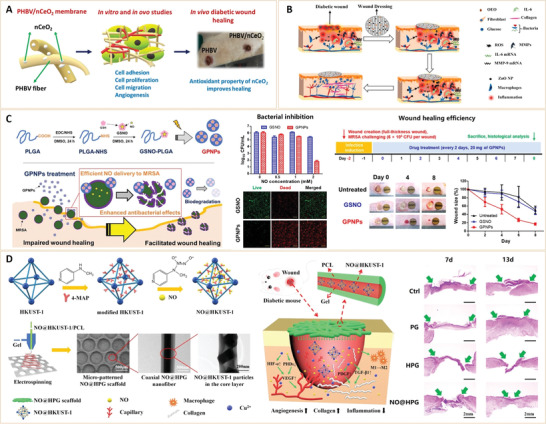
A) CeO2 NP incorporated electrospun poly(3‐hydroxybutyrate‐co‐3‐ hydroxyvalerate) membranes for diabetic wound healing. PHBV: poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate). Reproduced with permission.[ 305 ] Copyright 2020, American Chemical Society. B) Schematic illustration of the mechanism of action of the proposed wound dressing on the diabetic skin wound model. Reproduced with permission.[ 304 ] Copyright 2021, The Authors. Published by Elsevier. C) Nitric oxide‐releasing GSNO conjugated PLGA NPs for the treatment of MRSA‐infected cutaneous wounds. NO release profile of GPNPs in simulated wound fluid (SWF) at 37 °C; Antibacterial effects of GPNPs against MRSA compared to that of GSN. Green fluorescence (SYTO 9) and red fluorescence (propidium iodide) indicate live and dead bacteria, respectively; In vivo wound‐healing promotion effects of GPNPs in a MRSA‐challenged full‐thickness wound mouse model. GSNO: S‐nitrosoglutathione, PLGA: poly(lactic‐co‐glycolic acid), and MRSA: Staphylococcus aureus. Reproduced with permission.[ 309 ] Copyright 2020, MDPI. D) Schematic Illustration of the preparation of NO@HKUST‐1 and NO@HKUST‐1/PCL/Gel (NO@HPG) scaffold; Composite scaffold promotes the healing of diabetic wound. HKUST‐1, a kind of metal‐organic frameworks with copper ions as the metal center and trimesic acid (H3btc) as the organic ligand; PCL: hydrophobic polycaprolactone and Gel: gelatin. Reproduced with permission.[ 306 ] Copyright 2020, American Chemical Society.
