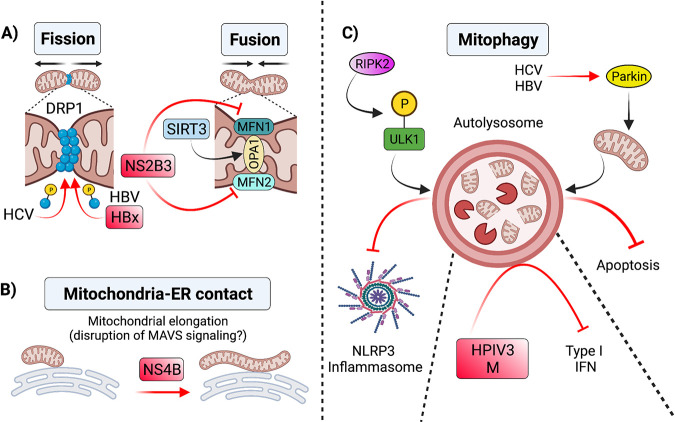
FIG 2.
Alterations to mitochondrial morphology during viral infection. (A) Changes in mitochondrial dynamics during viral infection have antiviral effects. For instance, SIRT3-mediated activation of OPA1 during HCMV infection promotes mitochondrial fusion, which interferes with viral replication (124). In contrast, viral infections can shift mitochondrial dynamics to favor replication and suppress innate defenses. For example, HCV (128) and HBV (129) induce phosphorylation and recruitment of DRP1 to mitochondria to enhance mitochondrial fission and subsequent mitophagy. Increased mitophagy driven by both viruses suppresses apoptosis and promotes viral persistence (128, 129). Dengue virus NS2B3 protein cleavage of MFN1/2 impairs mitochondrial fusion and inhibits type I IFN signaling (130). (B) Viruses induce changes in the interactions of mitochondria with other organelles that impact mitochondrial dynamics. For instance, dengue virus NS4B induces mitochondrial elongation, which alters MAM structure and dampens the immune response (134). (C) Mitophagy in antiviral responses. During influenza A infection in mice, RIPK2 mediated phosphorylation of ULK1 induces Parkin-independent mitophagy, which negatively regulates NLRP3 inflammasome activation. HCV and HBV also promote mitophagy by inducing translocation of Parkin to mitochondria (128, 129), which favors viral persistence by mitigating apoptosis. The M protein of the HPIV3 is also involved in mitophagy induction of infected cells, which results in inhibition of IFN-I response (136). SIRT3, sirtuin 3; OPA1, optic atrophy A1; HCV, hepatitis C virus; HBV, hepatitis B virus; DRP1, dynamin related protein 1; MAM, mitochondria-associated membrane; ULK1, Unc-51 like autophagy activating kinase 1; MFN1, mitofusin 1; MFN2, mitofusin 2; RIPK2, receptor interacting protein kinase 2; HPIV3, human parainfluenza virus. Figure not drawn to scale. Figure created with BioRender.com.