Abstract
Aims
The minute ventilation–carbon dioxide production relationship (VE/VCO2 slope) is widely used for prognostication in heart failure (HF) with reduced left ventricular ejection fraction (LVEF). This study explored the prognostic value of VE/VCO2 slope across the spectrum of HF defined by ranges of LVEF.
Methods and results
In this single‐centre retrospective observational study of 1347 patients with HF referred for cardiopulmonary exercise testing, patients with HF were categorized into HF with reduced (HFrEF, LVEF < 40%, n = 598), mid‐range (HFmrEF, 40% ≤ LVEF < 50%, n = 164), and preserved (HFpEF, LVEF ≥ 50%, n = 585) LVEF. Four ventilatory efficiency categories (VC) were defined: VC‐I, VE/VCO2 slope ≤ 29; VC‐II, 29 < VE/VCO2 slope < 36; VC‐III, 36 ≤ VE/VCO2 slope < 45; and VC‐IV, VE/VCO2 slope ≥ 45. The associations of these VE/VCO2 slope categories with a composite outcome of all‐cause mortality or HF hospitalization were evaluated for each category of LVEF. Over a median follow‐up of 2.0 (interquartile range: 1.9, 2.0) years, 201 patients experienced the composite outcome. Compared with patients in VC‐I, those in VC‐II, III, and IV demonstrated three‐fold, five‐fold, and eight‐fold increased risk for the composite outcome. This incremental risk was observed across HFrEF, HFmrEF, and HFpEF cohorts.
Conclusions
Higher VE/VCO2 slope is associated with incremental risk of 2 year all‐cause mortality and HF hospitalization across the spectrum of HF defined by LVEF. A multilevel categorical approach to the interpretation of VE/VCO2 slope may offer more refined risk stratification than the current binary approach employed in clinical practice.
Keywords: Cardiopulmonary exercise test, VE/VCO2 slope, Ventilatory efficiency, Heart failure, Heart failure hospitalization
Introduction
Cardiopulmonary exercise testing (CPET) measures cardiopulmonary reserve and provides an important prognostic tool in the management of heart failure (HF). In addition to peak oxygen consumption (peak VO2), the ratio of minute ventilation to carbon dioxide production (VE/VCO2 slope), a measure of ventilatory efficiency, strongly predicts adverse events in HF. 1 In clinical practice, a single VE/VCO2 slope threshold defining abnormal, most commonly ≥36, is employed across the spectrum of HF categorized by left ventricular ejection fraction (LVEF). 1 , 2 , 3 However, the predictive value of this VE/VCO2 slope threshold has largely been validated in patients with HF with reduced LVEF (HFrEF), 4 , 5 , 6 , 7 and its application in patients with HF with mid‐range LVEF (HFmrEF) and HF with preserved LVEF (HFpEF) requires additional validation. 8 , 9 , 10
Moreover, a wide range of VE/VCO2 slope values are seen in clinical practice, and so risk stratification based on a single cut‐point might underutilize the prognostic utility of VE/VCO2 slope. A multilevel categorization system developed in a small cohort (n = 448) of patients with HF, predominantly HFrEF (76%), correlated higher categories of VE/VCO2 slope cut‐points with increasing risk of cardiac‐related events. 3 Whether a similar classification could refine prognostication and clinical decision‐making warrants further evaluation in a larger cohort of patients with HF, especially those with HFmrEF or HFpEF. The objectives of this study were to evaluate and compare the prognostic utility of a multilevel VE/VCO2 slope classification system across categories of HF defined by LVEF.
Methods
Study design
This was a single‐centre, retrospective cohort study. Consecutive patients with a diagnosis of HF with known LVEF, who were clinically referred for CPET between June 2010 through December 2016 at Brigham and Women's Hospital, were considered for inclusion in this study. Patients were diagnosed as having HF if they had (i) a diagnosis of HF in the electronic medical record (EMR), (ii) documented use of loop diuretics/metolazone, or (iii) cardiomyopathy defined as LVEF < 40%. HF was then categorized into HFrEF (LVEF < 40%), HFmrEF (40% ≤ LVEF < 50%), and HFpEF (LVEF ≥ 50%) based on LVEF derived either by transthoracic echocardiography (n = 1309) or cardiac magnetic resonance imaging (n = 38) performed within a median of 1 day [interquartile range (IQR) 0, 76 days] of CPET.
Patients were excluded if they had incomplete CPET data, any history of a left‐ventricular‐assist device (LVAD) or cardiac transplantation prior to date of CPET, or where CPET did not follow a ramp protocol on an upright cycle ergometer. The final cohort consisted of 1347 patients (Supporting Information, Figure S1 ). This study was approved by the Partners Healthcare System Institutional Review Board.
Clinical information
Demographics, indications for exercise testing, and cardiovascular (CV) history and medications were prospectively recorded at the time of CPET by the exercise physiologist using a structured patient interview and EMR review. Ischaemic heart disease was defined as a history of myocardial infarction, coronary revascularization, or documented obstructive angiographic coronary artery disease. Hyperlipidaemia was defined as a known diagnosis of hyperlipidaemia or statin use at time of CPET. Diabetes mellitus was defined as a known diagnosis of diabetes mellitus or use of insulin or oral hypoglycaemic agents at time of CPET.
Cardiopulmonary exercise testing protocol
All CPETs were performed using a ramp protocol on an upright cycle ergometer (Lode Corival, Groningen, The Netherlands) with subjects breathing room air. Ventilatory expired gas analysis was performed using a metabolic cart (Breeze Suite 8.6.0.65 SP1, MGC Diagnostics, St. Paul, MN). Standard 12‐lead electrocardiogram and blood pressures were obtained at rest, every 2 to 3 min during exercise, and for a period ≥4 min during the recovery phase. Baseline metabolic evaluation was performed during a 2‐ min rest period before exercise and during cool‐down period for ≥1 min. VE, VO2, and VCO2 were acquired breath‐by‐breath and averaged for 10 s. Peak VO2 was defined as the highest 10 s averaged VO2 around the time of maximal effort. A set of previously published normative equations were used to estimate predicted peak VO2. 11 VE/VCO2 slope was calculated by linear regression from the start of freewheel to the end of the test (peak exercise). All gas exchange calculations were performed automatically by the metabolic cart software and were manually verified by the exercise physiologist performing the test and subsequently by a cardiologist. Four ventilatory categories (VC) were defined based on previous work by Arena et al. 3 : VC‐I, VE/VCO2 slope ≤ 29; VC‐II, 29 < VE/VCO2 slope < 36; VC‐III, 36 ≤ VE/VCO2 slope < 45; and VC‐IV, VE/VCO2 slope ≥ 45.
Study endpoints
The primary endpoint was a composite of all‐cause mortality or HF hospitalization (whichever occurred first) occurring within 2 years of CPET. Events were included through 1 July 2017. Mortality was determined using the Partners Health Care Research Patient Data Registry (linked to the Social Security Death Index, updated 30 July 2017). HF hospitalizations were abstracted by EMR review and were defined as unplanned admissions with clinical presentations consistent with HF exacerbations requiring escalation of existing HF treatment or initiation of new therapies (Table S1). Patients who received a LVAD or cardiac transplant after the CPET date were right‐censored at the time of this event. Patients who were lost to follow‐up were also right‐censored.
Statistical analyses
Continuous variables with approximately normal distributions are reported as means ± standard deviation and compared using one‐way ANOVA across all three HF cohorts. Continuous, non‐normal data are presented as medians with IQR and compared using the Kruskal–Wallis test. Categorical variables are presented as counts with percentages and compared using Fisher's exact test. Median follow‐up was estimated using the reverse Kaplan–Meier method. 12 To examine the association between VE/VCO2 slope category and the composite outcome, cause‐specific Cox proportional‐hazards regression models were used. The VE/VCO2 slope category ≤ 29 (VC‐I) was used as the reference group. A cause‐specific approach was used for modelling, as LVAD and cardiac transplant (n = 34) were treated as competing risks; therefore, both events were right‐censored. Unadjusted and adjusted (continuous age and gender) hazard ratios with 95% confidence intervals (CIs) were presented. Additionally, continuously measured VE/VCO2 slope was specified using restricted cubic splines with four knots in a model adjusting for age and gender. The number of knots was chosen using Akaike's information criterion. All models were assessed for overly influential patients, proportional hazards, and linearity. Cumulative incidence curves of the composite outcome were compared across VE/VCO2 slope categories for each LVEF cohort using Gray's test of equivalence.
Time‐dependent receiver operating characteristic analysis was performed using the nearest neighbours approach to examine the performance of previously defined VE/VCO2 slope cut‐offs (29 and 36) 3 as predictors of the composite outcome at 2 year follow‐up. 3 , 13 Sensitivity and specificity were calculated along with 95% CIs generated through bootstrapping using the percentile method. 14 We independently determined VE/VCO2 slope cut‐offs using Youden's index (maximizing sensitivity + specificity) and bootstrapped CIs to allow for comparison across LVEF.
Receiver operating characteristic analysis was also used to compare the performance of peak VO2, VE/VCO2 slope, and the combination of peak VO2 and VE/VCO2 slope as predictors of the 2 year composite outcome. All hypothesis testing was two‐tailed, and P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SAS Version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
Baseline demographics
The entire cohort of 1347 patients had a mean age of 58.0 ± 14.6 years and was predominantly Caucasian (85.0%) and male (60.5%) (Table 1 ). Compared with HFmrEF and HFpEF cohorts, patients in the HFrEF cohort were more likely to be male, with a higher prevalence of diabetes, hypertension, hypercholesterolemia, ischaemic heart disease, and smoking (Table 1 ). All CV medications were more commonly prescribed in HFrEF compared with other cohorts (Table 1 ).
TABLE 1.
Baseline demographics of the study cohorts
| Entire cohort (n = 1347) | HFrEF (n = 598) | HFmrEF (n = 164) | HFpEF (n = 585) | P value | |
|---|---|---|---|---|---|
| Gender, male | 815 (60.5%) | 430 (71.9%) | 98 (56.8%) | 287 (49.1%) | <0.0001 |
| Age, years | 58.0 ± 14.6 | 58.3 ± 13.1 | 55.8 ± 14.6 | 58.1 ± 16.0 | 0.12 |
| Body mass index (kg/m2) | 29.0 ± 6.3 | 28.2 ± 5.4 | 28.8 ± 6.2 | 29.9 ± 7.1 | <0.0001 |
| Race a | |||||
| Caucasian | 1076 (85.0%) | 475 (83.3%) | 130 (83.3%) | 471 (87.2%) | 0.59 |
| African American | 90 (7.1%) | 46 (8.1%) | 15 (9.6%) | 29 (5.4%) | |
| Hispanic/Latino | 35 (2.8%) | 20 (3.5%) | 5 (3.2%) | 10 (1.9%) | |
| Asian | 20 (1.6%) | 11 (1.9%) | 2 (1.3%) | 7 (1.3%) | |
| American Indian | 2 (0.2%) | 1 (0.2%) | 0 (0.0%) | 1 (0.2%) | |
| Others | 10 (0.8%) | 4 (0.7%) | 1 (0.6%) | 5 (0.9%) | |
| Missing/Unknown | 33 (2.6%) | 13 (2.3%) | 3 (1.9%) | 17 (3.2%) | |
| Left ventricular ejection fraction (%) | 42 ± 17 | 25 ± 7 | 42 ± 2 | 59 ± 6 | <0.0001 |
| Diabetes mellitus | 309 (22.9%) | 167 (27.9%) | 29 (17.7%) | 113 (19.3%) | <0.001 |
| Hypertension | 912 (67.7%) | 425 (71.1%) | 111 (67.7%) | 376 (64.3%) | 0.044 |
| Peripheral vascular disease | 21 (1.6%) | 13 (2.2%) | 3 (1.8%) | 5 (0.9%) | 0.18 |
| Hypercholesterolemia | 757 (56.2%) | 372 (62.2%) | 91 (55.5%) | 294 (50.3%) | <0.001 |
| Ischaemic heart disease | 388 (28.8%) | 244 (40.8%) | 37 (22.6%) | 107 (18.3%) | <0.0001 |
| Active smoking | 89 (6.6%) | 55 (9.2%) | 8 (4.9%) | 26 (4.4%) | 0.003 |
| ACEi/ARB | 772 (57.3%) | 422 (70.6%) | 108 (65.9%) | 242 (41.4%) | <0.0001 |
| Beta‐blocker | 1030 (76.5%) | 527 (88.1%) | 138 (84.2%) | 365 (62.4%) | <0.0001 |
| Aspirin | 617 (45.8%) | 303(50.7%) | 69 (42.1%) | 245 (41.9%) | 0.006 |
| Statin | 655 (48.6%) | 323 (54.0%) | 80 (48.8%) | 252 (43.1%) | <0.001 |
| Digoxin | 54 (4.0%) | 40 (6.7%) | 5 (3.1%) | 9 (1.5%) | <0.0001 |
| Loop diuretics/Metolazone | 849 (63.0%) | 403 (67.4%) | 89 (54.3%) | 357 (61.0%) | 0.004 |
| MRA | 327 (24.3%) | 214 (35.8%) | 45 (27.4%) | 68 (11.6%) | <0.0001 |
ACEi/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist.
Values are mean ± SD or n (%).
Available in 1266/1347 patients.
Cardiopulmonary exercise testing results
The mean peak VO2 for the entire cohort was 15.0 ± 6.6 mL/kg/min with a mean peak respiratory exchange ratio of 1.16 ± 0.14; more than half the cohort had peak VO2 < 14 mL/kg/min (Table 2 ). The median VE/VCO2 slope for the entire cohort was 32.0 (IQR 27.9, 38.4). Compared with patients with HFmrEF or HFpEF, patients with HFrEF had a higher proportion with peak VO2 below 14 mL/kg/min (60.9% vs. 45.1% vs. 49.0%, P < 0.001) and achieved a statistically significantly lower peak VO2 (13.8 ± 5.3 vs. 16.0 ± 6.8 vs. 16.2 ± 7.5, P < 0.001; Table 2 ). Patients with HFrEF also had higher median VE/VCO2 slope [33.8 (IQR 29.2, 41.8) vs. 30.0 (IQR 26.4, 44.5) vs. 31.0 (27.2, 36.8), P < 0.001; Table 2 ). In addition, patients with HFrEF had higher resting heart rate, lower peak heart rate, and lower systolic and diastolic blood pressures at rest and at peak exercise (Table 2 ). Compared with HFmrEF patients, patients with HFpEF had significantly higher resting and peak systolic blood pressures (Table 2 ).
TABLE 2.
Cardiopulmonary exercise testing results, overall, and by left ventricular ejection fraction category
| Entire cohort (n = 1347) | HFrEF (n = 598) | HFmrEF (n = 164) | HFpEF (n = 585) | P value | |
|---|---|---|---|---|---|
| VE/VCO2 slope | 32.0 (27.9, 38.4) | 33.8 (29.2, 41.8) | 30.0 (26.4, 44.5) | 31.0 (27.2, 36.8) | <0.001 |
| Peak VO2 (mL/kg/min) | 15.0 ± 6.6 | 13.8 ± 5.3 | 16.0 ± 6.8 | 16.0 ± 7.5 | <0.001 |
| Peak VO2 < 14 mL/kg/min | 721 (53.8%) | 363 (60.9%) | 73 (45.1%) | 285 (49.0%) | <0.001 |
| Peak RER | 1.16 ± 0.14 | 1.17 ± 0.14 | 1.16 ± 10.13 | 1.15 ± 0.14 | 0.005 |
| Resting heart rate (bpm) | 72.2 ± 14.0 | 73.8 ± 14.6 | 70.9 ± 12.5 | 70.8 ± 13.5 | <0.001 |
| Peak heart rate (bpm) | 120.4 ± 28.0 | 117.5 ± 26.6 | 123.1 ± 27.9 | 122.7 ± 29.2 | 0.003 |
| Resting SBP (mmHg) | 121.5 ± 21.9 | 114.1 ± 19.7 | 121.3 ± 17.9 | 128.7 ± 22.8 | <0.001 |
| Resting DBP (mmHg) | 73.3 ± 11.0 | 72.4 ± 10.4 | 73.3 ± 11.2 | 74.1 ± 11.4 | 0.040 |
| Peak SBP (mmHg) | 145.3 ± 31.5 | 132.7 ± 28.0 | 145.6 ± 25.2 | 158.2 ± 31.3 | <0.001 |
| Peak DBP (mmHg) | 73.6 ± 12.5 | 71.6 ± 11.9 | 74.6 ± 12.5 | 75.3 ± 12.8 | <0.001 |
| Exercise duration (min) | 7.4 ± 2.9 | 7.3 ± 2.8 | 7.9 ± 3.1 | 7.3 ± 2.9 | 0.05 |
bpm, beats per minute; DBP, diastolic blood pressure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection; HFrEF, heart failure with reduced ejection fraction; METs, metabolic equivalents; RER, respiratory exchange ratio; SBP, systolic blood pressure; VE/VCO2, minute ventilation to carbon dioxide production ratio; VO2, oxygen uptake.
Values are mean ± SD, median (interquartile range) or n (%).
VE/VCO2 slope category as a predictor of the 2 year composite endpoint
There were 201 composite events (65 deaths and 136 HF hospitalizations) over a median follow‐up of 2.0 (IQR: 1.9, 2.0) years (range: 6–730 days) from CPET (Table 3 ). Higher VE/VCO2 slope categories were associated with incremental 2 year cumulative incidences of the composite outcome within each HF category (Figure 1 ). Across the entire study cohort, compared with patients in VC‐I, those in VC‐II, III, and IV had over three‐fold, five‐fold, and eight‐fold increased hazards of the primary composite endpoint [hazard ratio (HR) 3.12, 95% CI 1.86 to 5.26, P < 0.001; HR 5.47, 95% CI 3.20 to 9.35, P < 0.001; HR 8.21, 95% CI 4.75 to 14.18, P < 0.001, respectively; Table 4 ].
TABLE 3.
Study outcome for the entire cohort and left ventricular ejection fraction subgroups
| Entire cohort (n = 1347) | HFrEF (n = 598) | HFmrEF (n = 164) | HFpEF (n = 585) | |
|---|---|---|---|---|
|
Follow‐up time a (years) Range, days |
2.0 (1.9, 2.0) 6–730 |
2.0 (2.0, 2.0) 6–730 |
2.0 (2.0, 2.0) 13–730 |
2.0 (1.8, 2.0) 8–730 |
| Deaths + HF hospitalizations, n | 201 | 128 | 19 | 54 |
| Deaths, n | 65 | 41 | 6 | 18 |
| HF hospitalizations, n | 136 | 87 | 13 | 36 |
HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection; HFrEF, heart failure with reduced ejection fraction.
Values are expressed as median (interquartile range).
Figure 1.
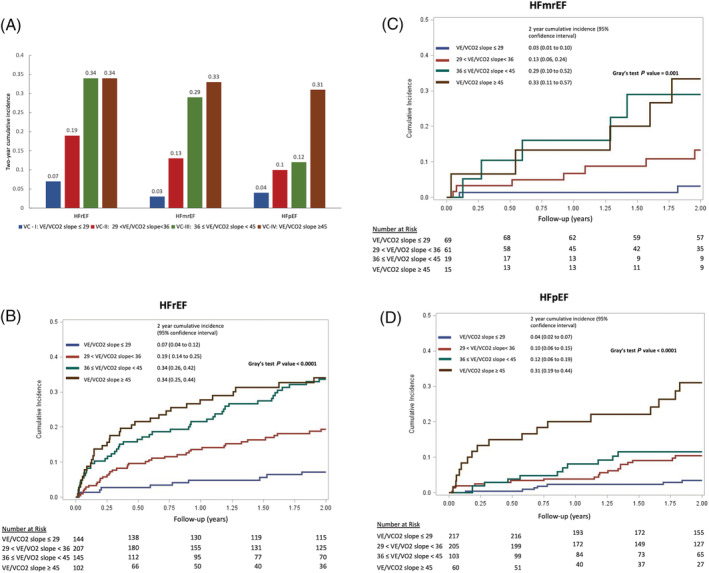
Cumulative incidence of 2 year composite outcome (death and HF admissions) across VE/VCO2 slope categories for (A) all cohorts, (B) HFrEF cohort over time, (C) HFmrEF cohort over time, and (D) HFpEF cohort over time. HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; VC, ventilatory efficiency category.
TABLE 4.
Association between VE/VCO2 slope categories and death + HF hospitalization across HF LVEF categories
| Entire cohort (n = 1347) | HFrEF (n = 598) | HFmrEF (n = 164) | HFpEF (n = 585) | |||||
|---|---|---|---|---|---|---|---|---|
| Adjusted HR a (95% CI) | P value | Adjusted HR a (95% CI) | P value | Adjusted HR a (95% CI) | P value | Adjusted HR a (95% CI) | P value | |
| VC‐I | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| VC‐II | 3.12 (1.86, 5.26) | <0.0001 | 2.82 (1.40, 5.68) | 0.004 | 3.98 (0.82, 19.23) | 0.09 | 2.67 (1.11, 6.40) | 0.028 |
| VC‐III | 5.47 (3.20, 9.35) | <0.0001 | 5.09 (2.55, 10.19) | <0.0001 | 8.72 (1.67, 45.60) | 0.010 | 2.96 (1.13, 7.78) | 0.027 |
| VC‐IV | 8.21 (4.75, 14.18) | <0.0001 | 6.02 (2.93, 12.38) | <0.0001 | 10.55 (2.03, 54.79) | 0.005 | 8.68 (3.52, 12.40) | <0.0001 |
CI, confidence interval; HF, heart failure; HFmrEF, HF with mid‐range ejection fraction; HFpEF, HF with preserved ejection; HFrEF, HF with reduced ejection fraction; HR, hazard ratio, VC, ventilatory category; VE/VCO2, minute ventilation to carbon dioxide production ratio.
VC‐I: VE/VCO2 slope ≤ 29, VC‐II: 29 < VE/VCO2 slope < 36, VC‐III: 36 ≤ VE/VCO2 slope < 45, VC‐IV: VE/VCO2 slope ≥ 45.
Adjusted for age (continuous) and gender.
In patients with HFrEF (n = 598), there were 128 composite events within 2 years of CPET (Table 3 ). Compared with patients in VC‐I, patients with HFrEF in VC‐II, III, and IV demonstrated incremental risk of the primary composite endpoint (HR 2.82, 95% CI 1.40 to 5.68, P = 0.004; HR 5.09, 95% CI 2.55 to 10.19, P < 0.001; HR 6.02, 95% CI 2.93 to 12.38, P < 0.001, respectively; Table 4 ).
Among patients with HFmrEF (n = 164), there were 19 composite events within 2 years of CPET (Table 3 ). Among patients with HFmrEF, VC‐III and IV demonstrated increased hazards of the primary composite endpoint compared with VC‐I in both unadjusted and adjusted analyses (Tables 4 and S2). VC‐II had a trend towards increased risk but did not achieve statistical significance in either unadjusted or adjusted analyses (Tables 4 and S2).
Fifty‐four composite events occurred within 2 years of CPET in the HFpEF cohort (n = 585) (Table 3 ). As with HFrEF, each incremental VE/VCO2 slope category was associated with an increased hazard of the composite endpoint, even after adjusting for age and gender (Tables 4 and S2).
When examined as a continuous variable across the entire cohort, increasing VE/VCO2 slope was associated with a progressive increase in the risk of the 2 year composite outcome (Figure 2 ).
Figure 2.
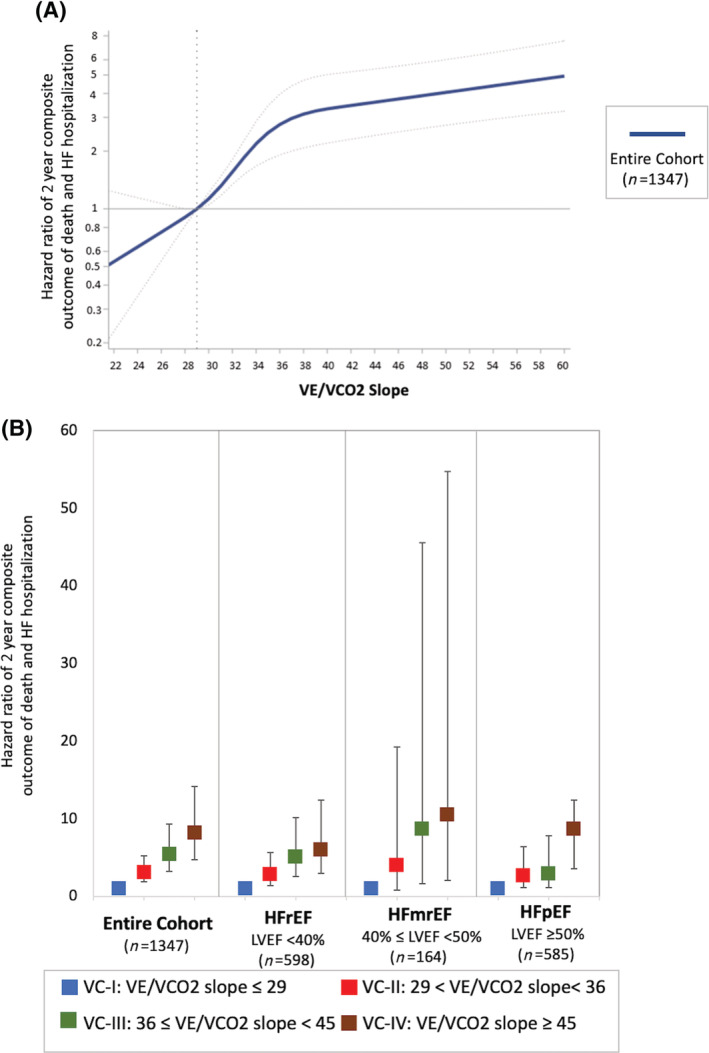
(A) A plot of continuously measured VE/VCO2 slope specified using restricted cubic splines by the hazard ratio for the 2 year composite outcome (death and HF admissions) (reference: VE/VCO2 slope = 29) is displayed for all HF strata (joint Wald test P value < 0.0001). (B) Incremental risk of the 2 year composite outcome across categories of increasing VE/VCO2 slope for the entire cohort and across the spectrum of HF defined by LVEF. HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Receiver operating characteristic analysis
Receiver operating characteristic analysis demonstrated that a threshold VE/VCO2 slope > 36, which is commonly employed in clinical practice, was more specific but less sensitive for the primary composite endpoint when applied to the HFmrEF and HFpEF cohorts compared with the HFrEF cohort (Table 5 ). Alternatively, a lower VE/VCO2 slope cut‐point of 29 was associated with higher sensitivity (>85.0% in all three categories) at the cost of lower specificity across all HF categories (Table 5 ). These cut‐points were then independently validated in our cohort (Figure 3 ). We compared the predictive value of peak VO2, VE/VCO2 slope, and the combination of peak VO2 and VE/VCO2 slope for each LVEF category. Our data showed overlapping C‐statistics for all three variables for each LVEF subgroup, suggesting that they had comparable prognostic value for the 2 year composite outcome (Table 6 and Figure S2).
TABLE 5.
Sensitivities and specificities of various VE/VCO2 slope cut‐points among HF groups, defined by LVEF
| VE/VCO2 slope ≥ 36 | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| HFrEF | 61.1% (52.6%, 70.7%) | 64.7% (60.5%, 69.2%) |
| HFmrEF | 41.6% (20.9%, 70.3%) | 82.1% (75.7%, 88.2%) |
| HFpEF | 47.0% (33.2%, 62.9%) | 74.2% (70.5%, 77.9%) |
| VE/VCO2 slope ≥ 29 | Sensitivity (95% CI) | Specificity (95% CI) |
| HFrEF | 90.5% (86.0%, 95.0%) | 28.4% (24.3%, 32.6%) |
| HFmrEF | 87.9% (67.1%, 100.0%) | 46.1% (38.0%, 55.7%) |
| HFpEF | 85.0% (75.0%, 93.8%) | 39.2% (34.9%, 43.3%) |
CI, confidence interval; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; VE/VCO2, minute ventilation to carbon dioxide production ratio.
Figure 3.
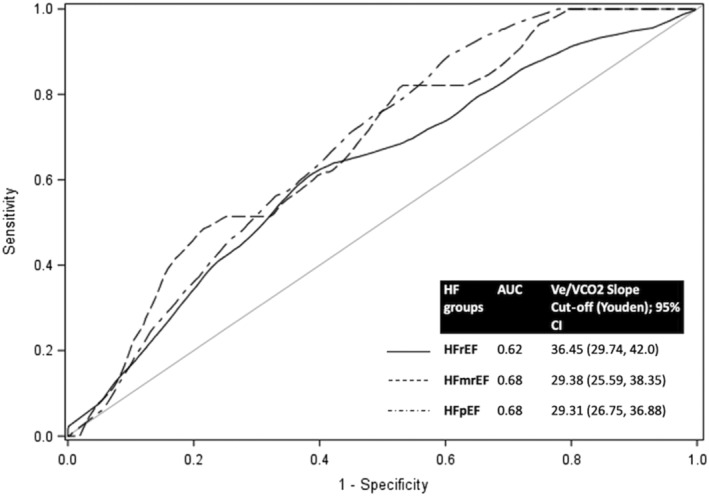
Time‐dependent receiver operating characteristic (ROC) curves describing the ability of VE/VCO2 slope to predict the 2 year composite outcome of death and HF admissions in patients with HFrEF, HFmrEF, and HFpEF, respectively. Optimal cut‐points were decided based on Youden's method. AUC, area under the curve; CI, confidence interval; HF, heart failure; HFmrEF, heart failure with mid‐range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
TABLE 6.
Concordance statistics to evaluate predictive value of VE/VCO2 slope, peak VO2, and the combination of VE/VCO2 slope and peak VO2 in assessing risk of death + HF hospitalization across HF LVEF categories
| C‐statistic | |||
|---|---|---|---|
| VE/VCO2 slope 95% CI | Peak VO2 95% CI | VE/VCO2 slope + peak VO2 95% CI | |
| HFrEF | 0.67 (0.63, 0.71) | 0.71 (0.67, 0.75) | 0.73 (0.69, 0.77) |
| HFmrEF | 0.72 (0.60, 0.84) | 0.85 (0.78, 0.92) | 0.86 (0.80, 0.92) |
| HFpEF | 0.71 (0.64, 0.78) | 0.79 (0.74, 0.84) | 0.80 (0.75, 0.85) |
CI, confidence interval; HF, heart failure; HFmrEF, HF with mid‐range ejection fraction; HFpEF, HF with preserved ejection; HFrEF, HF with reduced ejection fraction; HR, hazard ratio; peak VO2, peak oxygen consumption; VE/VCO2, minute ventilation to carbon dioxide production ratio.
Discussion
In this retrospective cohort study, we explored the prognostic value of a multilevel classification system for VE/VCO2 slope to predict the 2 year composite outcome of all‐cause mortality and HF hospitalization across HF cohorts defined by LVEF. Incremental decreases in ventilatory efficiency were associated with an increased risk of the composite outcome in patients with HFrEF, HFmrEF, and HFpEF, respectively. The currently used cut‐point of VE/VCO2 slope ≥ 36 was prognostic across all HF categories but was associated with lower sensitivity for predicting adverse outcomes in the HFmrEF or HFpEF cohorts compared with patients with HFrEF. Furthermore, among patients with HFrEF or HFpEF, a VE/VCO2 slope between 29 and 36, currently considered borderline abnormal in clinical practice, was associated with a greater than two‐fold risk of the composite outcome, after adjusting for age and gender. Thus, our data suggest that relying on a single VE/VCO2 slope threshold of ≥36 in HF patients may under‐acknowledge risk in patients with VE/VCO2 slopes in this ‘borderline abnormal’ category who might otherwise benefit from closer follow‐up and intensification of medical therapy.
Studies examining VE/VCO2 slope as a predictor of adverse outcomes have largely focused on patients with HFrEF. 4 , 5 , 6 , 7 , 15 , 16 , 17 , 18 , 19 , 20 , 21 In patients with HFrEF, every unit increase in VE/VCO2 slope has been associated with a 4–10% increase in the risk of all‐cause mortality, CV death, or cardiac transplantation. 15 , 16 , 18 , 20 VE/VCO2 slope has received comparatively less attention in patients with HFpEF and HFmrEF. 9 , 10 , 20 , 21 , 22 One small study (n = 173) failed to establish a significant association between increasing VE/VCO2 slope and the risk of death or cardiac transplantation in patients with HFpEF. 9 However, another study of 88 patients with LVEF ≥ 45% reported an almost two‐fold increased risk of all‐cause mortality with each 10 unit increase in VE/VCO2 slope. 21 Similar results were seen in a large retrospective study of 493 patients with LVEF ≥ 50% where each unit increase in VE/VCO2 slope was associated with a 1.5‐fold increase in the risk of CV hospitalization and death. 22 Data in HFmrEF are more limited, and a single study of 144 patients reported a 12% increase in the combined risk of LVAD implantation, cardiac transplantation, or all‐cause mortality per unit increase in VE/VCO2 slope. 20 A recent analysis of 269 patients with HF across a range of LVEF similarly showed that increasing VE/VCO2 slope categories were associated with an increased risk of HF hospitalization and death. However, this analysis did not assess the predictive value of different VE/VCO2 slope categories in patients with HFrEF, HFmrEF, and HFpEF. 23 Our study adds to this literature and advances our understanding of the prognostic value of VE/VCO2 slope in patients with HFmrEF and HFpEF, reinforcing the utility of this parameter in these cohorts.
In current clinical practice, VE/VCO2 slope is categorized as normal/abnormal in a dichotomous manner across a cut‐point of 36. This binary approach fails to provide optimal refinement of risk prediction. Prior studies attempting to categorize VE/VCO2 slope as a multilevel variable have shown value in patients with HFrEF but have conflicting results in patients with HFpEF. No prior studies have evaluated VE/VCO2 slope as a multilevel variable in HFmrEF. Francis et al. divided 303 patients with mean LVEF 25 ± 11% into four quartiles based on VE/VCO2 slope and demonstrated that patients in higher quartiles had increasing risk of 2 year mortality (3%, 17%, 26%, and 49% from lowest to highest quartiles, respectively). 24 In a cohort of 663 patients with HFrEF, Ferreira et al. categorized VE/VCO2 slope into five‐unit increments above 30 and found worse survival across ascending VE/VCO2 slope categories. 25 Similarly, Arena et al. divided 448 patients with either systolic or diastolic HF into four VE/VCO2 slope categories and observed that higher VE/VCO2 slope categories were associated with a higher likelihood of death, cardiac transplantation, or LVAD implantation; in subgroup analyses, these results held true in patients with HFrEF (LVEF ≤ 40%) but not in those with EF > 40%. 3 In contrast, two studies have shown incremental risk with increasing VE/VCO2 slope in patients with HFpEF. A study of 88 patients with HFpEF reported 1.44‐fold and 3.57‐fold increased risk of all‐cause mortality among those in the middle and highest tertiles for VE/VCO2 slope compared with those in the lowest tertile, respectively. 21 A recent study of 483 patients with HFpEF (LVEF ≥ 50%) similarly demonstrated that patients in the middle and highest tertiles for VE/VCO2 slope had a 1.72‐fold and 2.44‐fold increased risk of CV hospitalization and death compared with those in the lowest tertile. 22 A study of 269 subjects showed a 1.4‐fold increase risk of HF hospitalization and death with increasing VE/VCO2 slope categories across a range of LVEF. 23 Our study builds upon the available data and highlights an opportunity for risk refinement by considering VE/VCO2 slope as a multilevel rather than dichotomous variable across all types of HF defined by LVEF.
Our findings demonstrate that the current VE/VCO2 slope cut‐point of ~36 is less sensitive for predicting risk in patients with HFmrEF and HFpEF compared with those with HFrEF. Thus, our data caution against the uniform application of this cut‐point in HFmrEF and HFpEF patients and larger studies are needed to identify optimal VE/VCO2 slope thresholds in these cohorts. Our data also highlight that a borderline VE/VCO2 slope of 29–36 manifests significantly increased risk in patients with HFrEF and HFpEF relative to a normal VE/VCO2 slope of less than 29. While not statistically significant, a similar trend for increased risk was seen in patients with HFmrEF. This increased risk should be considered when managing patients with VE/VCO2 slope of 29–36.
Our study has several limitations inherent to the retrospective study design. These include the selection bias of studying patients with HF clinically referred for CPET, as reflected by the relatively younger age of patients with HFpEF in this study compared with community studies. The small number of events (n = 19) in the HFmrEF cohort may have limited the power to detect a significant predictive value for VE/VCO2 slope of 29–36. Importantly, we are unable to account for the effect of non‐HF comorbidities that influence VE/VCO2 slope, such as non‐group II pulmonary hypertension or chronic obstructive pulmonary disease, on risk of adverse events across different HF categories. HFpEF was defined as a chart diagnosis of HF or documented use of loop diuretics/metolazone plus EF ≥ 50% defined by echocardiography or cardiac magnetic resonance imaging. We did not have data on natriuretic peptides or detailed echocardiogram characteristics required to make an accurate diagnosis of HFpEF. Furthermore, we acknowledge that HFpEF is a heterogeneous disease entity. However, given the nature of retrospective data, we were unable to define the particular aetiology of HFpEF for each subject in our study. Additionally, our cohort was relatively young, and our findings may not be applicable to those with age‐associated HFpEF.
In conclusion, the current clinical practice of utilizing a single threshold for defining an abnormal VE/VCO2 slope in all patients with HF should be reconsidered. This approach is limited by differences in sensitivity for risk prediction across different HF categories defined by LVEF. Additionally, we demonstrate that a multilevel, rather than dichotomous, approach to VE/VCO2 slope interpretation further refines risk prediction across all categories of HF defined by LVEF. Specifically, in patients with HFrEF or HFpEF, a VE/VCO2 slope between 29 and 36, currently considered borderline, is associated with increased risk and should be considered when managing patients in clinical practice. Larger studies are needed to develop more granular systems for the interpretation of VE/VCO2 slope within each of the HF categories, defined by LVEF, to maximally inform risk stratification and optimize patient management.
Conflict of interest
Outside the submitted work, J.D.G. received research support from Amgen, Inc. A.N. is a consultant for Takeda Oncology, AstraZeneca and Boehringer Ingelheim and receives research support from Amgen, Inc. A.O. has received research support from and serves on an Independent Data Monitoring Committee for Actelion/Johnson and Johnson. J.G., J.C., R.C., C.B., J.H., M.R.M., B.A.M., and M.D.C. have no conflicts relevant to the manuscript to disclose.
Funding
This work was supported by the Goodman Master Clinician Award, Brigham and Women's Hospital, Boston, MA granted to J.G. in 2017–2019. Additional sources of funding include the Gelb Master Clinician Award and the Catherine Geoff Fitch Fund, Brigham and Woman's Hospital Boston, MA granted to A.N. M.R.M. reports no directly relevant conflicts to the current work but would like to disclose other potential conflicts including receiving travel support and consulting fees, paid to Brigham and Women's Hospital, from Abbott, fees for serving on a steering committee from Medtronic and Janssen (Johnson & Johnson), fees for serving on a data and safety monitoring board from Mesoblast, consulting fees from Portola, Bayer, Triple gene, Baim Institute of Clinical Research, and fees for serving as a scientific board member from NuPulseCV, Leviticus, and FineHeart.
Supporting information
Figure S1. CONSORT diagram of the study cohort.
Figure S2A. Time‐dependent Receiver Operating Characteristic (ROC) Curves describing the ability of VE/VCO2 slope, peak VO2 and the combination of VE/VCO2 slope and peak VO2 to predict the two‐year composite outcome of all‐cause mortality and HF hospitalizations in patients with HFrEF.
Figure S2B. Time‐dependent Receiver Operating Characteristic (ROC) Curves describing the ability of VE/VCO2 slope, peak VO2 and the combination of VE/VCO2 slope and peak VO2 to predict the two‐year composite outcome of all‐cause mortality and HF hospitalizations in patients with HFmrEF.
Figure S2C. Time‐dependent Receiver Operating Characteristic (ROC) Curves describing the ability of VE/VCO2 slope, peak VO2 and the combination of VE/VCO2 slope and peak VO2 to predict the two‐year composite outcome of all‐cause mortality and HF hospitalizations in patients with HFpEF.
Table S1. Definition of heart failure admission.
Table S2. Unadjusted association Between VE/VCO2 Slope Categories and Death + HF Hospitalization Across HF Categories.
Gong, J. , Castro, R. R. T. , Caron, J. P. , Bay, C. P. , Hainer, J. , Opotowsky, A. R. , Mehra, M. R. , Maron, B. A. , Di Carli, M. F. , Groarke, J. D. , and Nohria, A. (2022) Usefulness of ventilatory inefficiency in predicting prognosis across the heart failure spectrum. ESC Heart Failure, 9: 293–302. 10.1002/ehf2.13761.
John D. Groarke and Anju Nohria contributed equally to this work.
References
- 1. Malhotra R, Bakken K, D'Elia E, Lewis GD. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail 2016; 4: 607–616. [DOI] [PubMed] [Google Scholar]
- 2. Mehra MR, Canter CE, Hannan MM, Semigran MJ, Uber PA, Baran DA, Danziger‐Isakov L, Kirklin JK, Kirk R, Kushwaha SS, Lund LH, Potena L, Ross HJ, Taylor DO, Verschuuren EAM, Zuckermann A, International Society for Heart Lung Transplantation (ISHLT) Infectious Diseases, Pediatric and Heart Failure and Transplantation Councils . The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10‐year update. J Heart Lung Transplant 2016; 35: 1–23. [DOI] [PubMed] [Google Scholar]
- 3. Arena R, Myers J, Abella J, Peberdy MA, Bensimhon D, Chase P, Guazzi M. Development of a ventilatory classification system in patients with heart failure. Circulation 2007; 115: 2410–2417. [DOI] [PubMed] [Google Scholar]
- 4. Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. Am Heart J 2004; 147: 354–360. [DOI] [PubMed] [Google Scholar]
- 5. Keteyian SJ, Patel M, Kraus WE, Brawner CA, McConnell T, Piña IL, Leifer ES, Fleg JL, Blackburn G, Fonarow GC, Chase PJ, Piner L, Vest M, O'Connor CM, Ehrman JK, Walsh MN, Ewald G, Bensimhon D, Russell SD, HF‐ACTION Investigators . Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol 2016; 67: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corrà U, Mezzani A, Bosimini E, Scapellato F, Imparato A, Giannuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J 2002; 143: 418–426. [DOI] [PubMed] [Google Scholar]
- 7. Kleber FX, Vietzke G, Wernecke KD, Bauer U, Opitz C, Wensel R, Sperfeld A, Gläser S. Impairment of ventilatory efficiency in heart failure: prognostic impact. Circulation 2000; 101: 2803–2809. [DOI] [PubMed] [Google Scholar]
- 8. Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol 2005; 46: 1883–1890. [DOI] [PubMed] [Google Scholar]
- 9. Shafiq A, Brawner CA, Aldred HA, Lewis B, Williams CT, Tita C, Schairer JR, Ehrman JK, Velez M, Selektor Y, Lanfear DE, Keteyian SJ. Prognostic value of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. The Henry Ford HospITal CardioPulmonary EXercise Testing (FIT‐CPX) project. Am Heart J 2016; 174: 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan J, Gong SJ, Li L, Yu HY, Dai HW, Chen J, Tan CW, Xv QH, Cai GL. Combination of B‐type natriuretic peptide and minute ventilation/carbon dioxide production slope improves risk stratification in patients with diastolic heart failure. Int J Cardiol 2013; 162: 193–198. [DOI] [PubMed] [Google Scholar]
- 11. Wasserman K, Hansen J, Sue D, Casaburi R, Whipp BJ. Principles of Exercise Testing and Interpretation. Baltimore: Lippincott, Williams & Wilkins; 2004. [Google Scholar]
- 12. Clark TG, Bradburn MJ, Love SB, Altman DG. Survival analysis part I: basic concepts and first analyses. Br J Cancer 2003; 89: 232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamarudin AN, Cox T, Kolamunnage‐Dona R. Time‐dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol 2017; 17: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Efron B, Tibshirani R. An Introduction to the Bootstrap. CRC Press; 1994. [Google Scholar]
- 15. Sarullo FM, Fazio G, Brusca I, Fasullo S, Paterna S, Licata P, Novo G, Novo S, di Pasquale P. Cardiopulmonary exercise testing in patients with chronic heart failure: prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc Med J 2010; 4: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chua TP, Ponikowski P, Harrington D, Anker SD, Webb‐Peploe K, Clark AL, Poole‐Wilson PA, Coats AJS. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol 1997; 29: 1585–1590. [DOI] [PubMed] [Google Scholar]
- 17. Gitt AK, Wasserman K, Kilkowski C, Kleemann T, Kilkowski A, Bangert M, Schneider S, Schwarz A, Senges J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002; 106: 3079–3084. [DOI] [PubMed] [Google Scholar]
- 18. Nanas SN, Nanas JN, Sakellariou DC, Dimopoulos SK, Drakos SG, Kapsimalakou SG, Mpatziou CA, Papazachou OG, Dalianis AS, Anastasiou‐Nana MI, Roussos C. VE/VCO2 slope is associated with abnormal resting haemodynamics and is a predictor of long‐term survival in chronic heart failure. Eur J Heart Fail 2006; 8: 420–427. [DOI] [PubMed] [Google Scholar]
- 19. Tsurugaya H, Adachi H, Kurabayashi M, Ohshima S, Taniguchi K. Prognostic impact of ventilatory efficiency in heart disease patients with preserved exercise tolerance. Circ J 2006; 70: 1332–1336. [DOI] [PubMed] [Google Scholar]
- 20. Nadruz W, West E, Sengeløv M, Santos M, Groarke JD, Forman DE, Claggett B, Skali H, Shah AM. Prognostic value of cardiopulmonary exercise testing in heart failure with reduced, midrange, and preserved ejection fraction. J Am Heart Assoc 2017; 6: e006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klaassen SHC, Liu LCY, Hummel YM, Damman K, van der Meer P, Voors AA, Hoendermis ES, van Veldhuisen DJ. Clinical and hemodynamic correlates and prognostic value of VE/VCO. J Card Fail 2017; 23: 777–782. [DOI] [PubMed] [Google Scholar]
- 22. Nayor M, Xanthakis V, Tanguay M, Blodgett JB, Shah RV, Schoenike M, Sbarbaro J, Farrell R, Malhotra R, Houstis NE, Velagaleti RS, Moore SA, Baggish AL, O'Connor GT, Ho JE, Larson MG, Vasan RS, Lewis GD. Clinical and hemodynamic associations and prognostic implications of ventilatory efficiency in patients with preserved left ventricular systolic function. Circ Heart Fail 2020; 13: e006729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guazzi M, Borlaug B, Metra M, Losito M, Bandera F, Alfonzetti E, Boveri S, Sugimoto T. Revisiting and implementing the weber and ventilatory functional classifications in heart failure by cardiopulmonary imaging phenotyping. J Am Heart Assoc 2021; 10: e018822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, Coats AJ. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J 2000; 21: 154–161. [DOI] [PubMed] [Google Scholar]
- 25. Ferreira AM, Tabet JY, Frankenstein L, Metra M, Mendes M, Zugck C, Beauvais F, Cohen‐Solal A. Ventilatory efficiency and the selection of patients for heart transplantation. Circ Heart Fail 2010; 3: 378–386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CONSORT diagram of the study cohort.
Figure S2A. Time‐dependent Receiver Operating Characteristic (ROC) Curves describing the ability of VE/VCO2 slope, peak VO2 and the combination of VE/VCO2 slope and peak VO2 to predict the two‐year composite outcome of all‐cause mortality and HF hospitalizations in patients with HFrEF.
Figure S2B. Time‐dependent Receiver Operating Characteristic (ROC) Curves describing the ability of VE/VCO2 slope, peak VO2 and the combination of VE/VCO2 slope and peak VO2 to predict the two‐year composite outcome of all‐cause mortality and HF hospitalizations in patients with HFmrEF.
Figure S2C. Time‐dependent Receiver Operating Characteristic (ROC) Curves describing the ability of VE/VCO2 slope, peak VO2 and the combination of VE/VCO2 slope and peak VO2 to predict the two‐year composite outcome of all‐cause mortality and HF hospitalizations in patients with HFpEF.
Table S1. Definition of heart failure admission.
Table S2. Unadjusted association Between VE/VCO2 Slope Categories and Death + HF Hospitalization Across HF Categories.


