Figure 1.
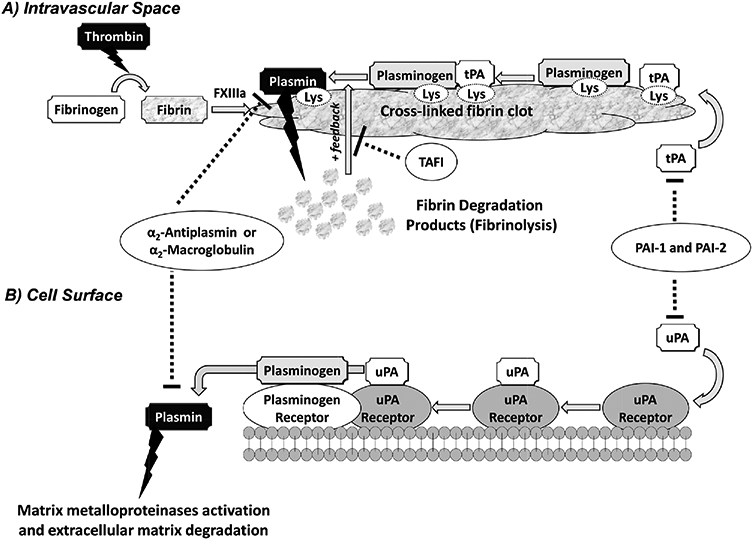
A simplified schematic representation of the plasminogen-plasmin system. Plasminogen is activated in the intravascular space by tissue plasminogen activator (tPA) (A) or at the cell surface by urokinase plasminogen activator (uPA) (B). (A) During coagulation, thrombin converts fibrinogen into soluble fibrin monomers, which cross-link by the action of factor XIIIa resulting in formation of insoluble cross-linked fibrin clot. If the fibrinolysis is to be initiated, plasminogen and tPA bind to fibrin through their lysine-binding sites (LBSs) present on the kringle domains. Formation of such ternary complex activates plasminogen and releases plasmin, which hydrolyzes fibrin. In a positive feedback mechanism, plasmin promotes its own formation by exposing more C-terminal lysine residues of fibrin. Four physiologic inhibitors regulate fibrinolysis including plasminogen-activator inhibitor −1 and −2 (PAI-1 and PAI-2), which inhibit tPA and uPA, and α2-antiplasmin and α2-macroglobulin, which inactivate any unbound plasmin. In addition, activated thrombin-activatable fibrinolysis inhibitor, which upon activation by thrombin removes the C-terminal lysine residues of fibrin, also prevents plasmin generation. (B) Activator uPA binds to its receptor at the cell surface and activates plasminogen that is bound to its receptor nearby. This releases plasmin into the extracellular matrix. Plasmin generated at the cell surface is primarily regulated by the action of α2-antiplasmin, PAI-1, and -2. Plasmin generated at the cell surface plays key roles in MMP activation and ECM degradation.
