Abstract
We recorded electroencephalogram (EEG) and simultaneously documented the state of both eyelids during sleep and wakefulness in a sub-adult male white whale over a 4-day-period. We showed that the white whale was the fifth species of Cetaceans, which exhibits unihemispheric slow wave sleep. We found that the eye contralateral to the sleeping hemisphere in this whale was usually closed (right eye, 52% of the total sleep time in the contralateral hemisphere; left eye, 40%) or in an intermediate state (31 and 46%, respectively) while the ipsilateral eye was typically open (89 and 80%). Episodes of bilateral eye closure in this whale occupied less than 2% of the observation time and were usually recorded during waking (49% of the bilateral eye closure time) or low amplitude sleep (48%) and rarely in high amplitude sleep (3%). In spite of the evident overall relationship between the sleeping hemisphere and eye state, EEG and eye position in this whale could be independent over short time periods (less than 1 min). Therefore, eye state alone may not accurately reflect sleep state in Cetaceans. Our data support the idea that unihemispheric sleep allows Cetaceans to monitor the environment.
Keywords: Unihemispheric sleep, Slow wave sleep, Unilateral eye closure, Eye state, Beluga, White whale, Cetaceans
Behavioral observations indicated that dolphins exhibit unilateral eye closure during periods of rest and it was speculated that the hemisphere contralateral to the closed eye might be asleep [2,3,5,7,10,13]. Subsequently, bilateral electroencephalogram (EEG) recording demonstrated that slow wave sleep (SWS) in dolphins could be low or high voltage unihemispheric, bilateral but asymmetric, bilateral at low voltage, and rarely bilateral at high voltage, according to EEG criteria [8,9,11,12]. Some electrophysiological data ([10], S. Ridgway, personal communication) suggest that a relation between the EEG in the two hemispheres and the state of the eyes is present in Cetaceans. However, the nature of the eyelid position during these states has not been established because of the difficulty of reliably documenting the position of the eyelids in swimming dolphins. In this report we present the first evidence that the state of the eyes in Cetaceans is closely associated with the EEG activity in the two hemispheres.
We recorded EEG, electrocardiogram (ECG) and simultaneously documented the state of both eyelids during sleep and wakefulness in a sub-adult male white whale (Delphinapterus leucas; weight 535 kg and length 3.2 m) continuously over the 4 days. The white whale or beluga is a representative of Odontoceti whales. The belugas are circumpolar, mainly arctic and subarctic animals. They have a number of physiological and behavioral adaptations allowing them to survive in the ice habitat including an ability to sleep under the water surface during long respiratory pauses [6]. Four EEG subdural electrodes were implanted in this whale under local anesthesia bilaterally in holes drilled through the skull over symmetrical occipital and parietal locations of the two hemispheres, using the minimally disruptive surgical procedure described in detail elsewhere [9,11,12]. ECG was recorded from two 5-mm-long sterile 25-gauge hypodermic needles placed under the skin from each side of the beluga’s dorsal ridge. After implantation the whale was placed in a pool (4×4×1.2 m) filled with seawater. Movement of the whale was restricted by a harness made from a soft fishing net attached to three belts. These belts were fastened behind the beluga’s blowhole, at the level of the dorsal ridge, and in front of the fluke. Before the surgery the whale was adapted to the harness for 2 weeks. During the recording we secured the harness to the corners of the pool by four ropes. In this situation the whale stayed most of the time near the surface in horizontal position. It was capable of swimming in place (forward, backward, up and down) for a distance of about 50 cm but not around the pool. This allowed us to observe on a monitor and videotape in real time the state of both eyes using four high sensitive cameras (0.1–0.3 lx, made by Panasonic and Samsung). The cameras were positioned on two sides of the pool and underwater and were connected to a video recorder (Panasonic AG 6730E) via a multiplexor (Panasonic WJ-FS20). Bipolar EEG recordings from the two hemispheres and ECG were band pass filtered (0.3–30.0 Hz), amplified, digitally sampled (100 Hz sampling rate) and stored using a CED1401 plus and Spike 2.24 Software (Cambridge Electronic Design, Cambridge, UK). The water in the pool was changed twice a day (between 08:00 and 09:00 h and between 19:00 and 20:00 h), which took approximately 30 min, and after this the whale was fed fish. Illumination was natural with the light period approximately between 08:00 and 18:00 h. During the night the poll area was illuminated by electric lamps (approximately 30–50 lx at the level of the animal’s head). The water temperature in the pool during varied between 16 and 19 °C.
We scored EEG from the two hemispheres visually in 30-s epochs (desynchronization; low and high amplitude SWS [8,9,12]) and did EEG spectral analysis in 5-s epochs. We observed a number of muscle jerks in this whale, which occurred both during bilateral desynchronized and unilateral synchronized EEG. However, the data we have is not sufficient to reach any conclusion on the presence of paradoxical (REM) sleep in this whale. Therefore, all epochs with bilateral desynchronization of the EEG were considered to represent wakefulness and all others SWS.
We used two approaches to analyze the relationship between eye state and EEG in this whale. In the first (‘on-line sampling’), we programmed a video multiplexer to project on a monitor video images of each eye, alternating at 5-s intervals. When both eyes were clearly visible an observer was continuously watching these alternating images of the eyes. The state of each eye was scored as open when the eye was open 3 s or longer per epoch (5 s) and closed when the eye was fully closed for the whole epoch. All other states were scored as intermediate (e.g. the eye was half closed or several blinks were seen). At the end of each 5-s epoch (when the images switched) the eye state at the ended epoch was coded by pressing one of six designated keyboard keys (three for each eye) and stored by Spike 2.24 Software together with EEG and ECG. During analysis the state of each eye was extrapolated for a 30-s epoch (constituting three alternating 5-s epochs for each eyes). The following rule was used: eye state in the overall 30-s epoch was scored as open if two to three out of three 5-s epochs were scored as open; as closed if two out of three epochs were scored as closed and one as intermediate or all three epochs were scored as closed; and as intermediate in all other cases. A total of 20 h of data related to the state of two eyes were collected over 2 consecutive days; 11 h of these constituted sleep.
The second approach (‘replay sampling’) involved reviewing videotapes and scoring the state of the two eyes simultaneously in 5-s epochs using the criteria described above. The EEG spectral power from each hemisphere was calculated in 5-s epochs and synchronized with the state of the eyes. We have analyzed two 3-h uninterrupted sleep periods when both eyes were clearly visible for the entire time and one of them is presented in Fig. 1. A 3-h sleep period analyzed using both methods showed a high correlation between the two approaches in terms of their estimations of the amount of time the whale spent in each eyes state (correlation between the state of the eyes in 30-s epochs was 83% for the left eye and 87% for the right eye).
Fig. 1.
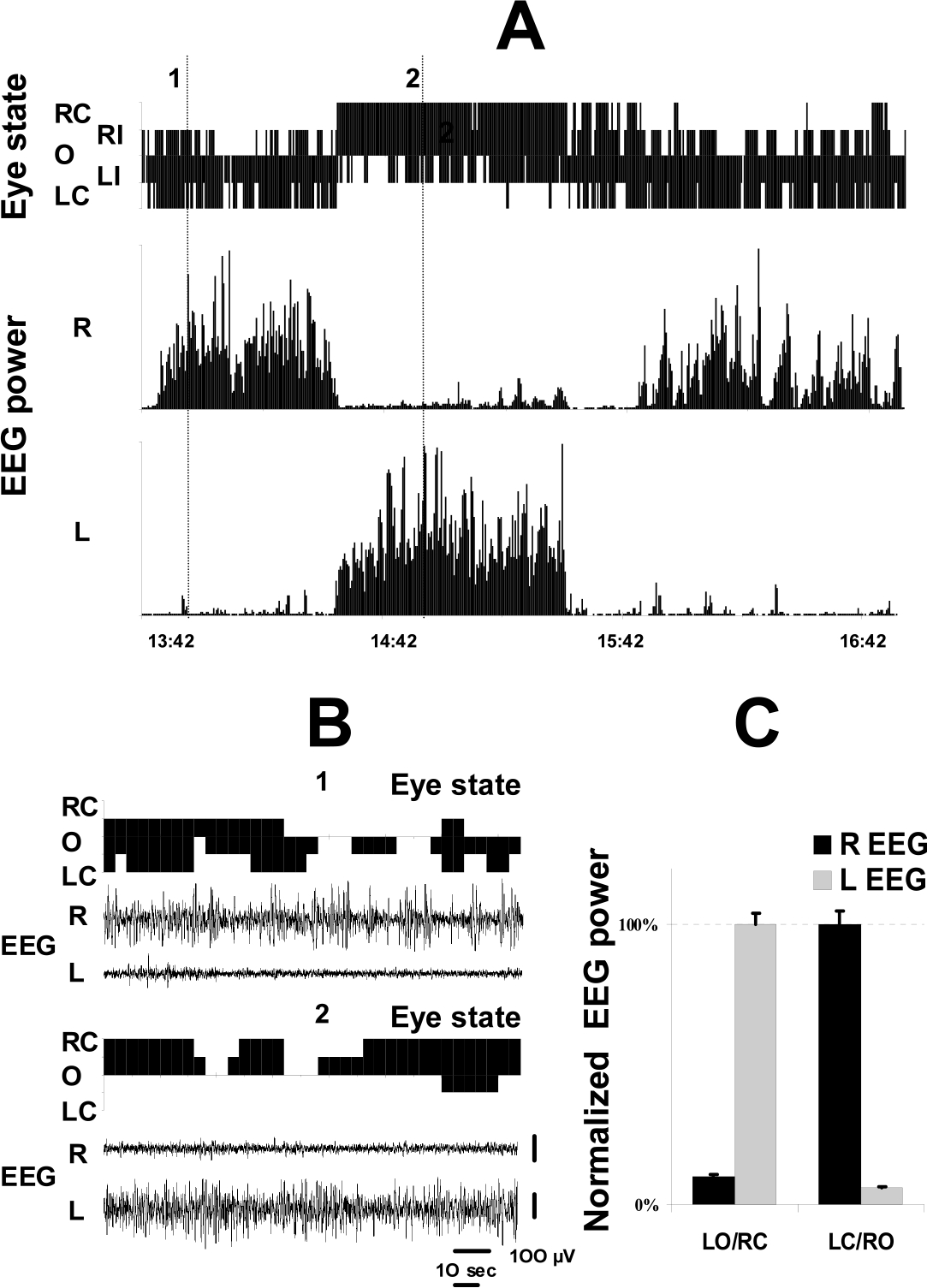
Relationship between EEG and the state of eyes in a white whale. (A) The state of eyelids and EEG spectral power (1–3 Hz; 5-s epochs) from the two hemispheres (R, right; L, left) in a white whale recorded over a 3-h period. EEG power was normalized as a percentage of the maximal power in each hemisphere during this period. The state of each eye (R, right; L, left) was scored in real time (O, open; I, intermediate; or C, closed) and then categorized for 5-s epochs as described in the text. Note that this compressed figure does not show short lasting changes in eye state. (B) Expansion of the two 2.5-min recordings of the EEG and the state of both eyes. The examples show the EEG asynchrony and parallel changes in eye state recorded in this whale at the times marked as 1 and 2 in Fig. 1A. Note that the EEG does not change immediately with changes in eye position. The right eye did not close during episode 1 and the left eye did not close during episode 2. (C) The average EEG spectral power in the two hemispheres during episodes with unilateral eye opening (LO/RC, left open and right closed; LC/RO, left closed and right open). EEG power was normalized as a percent of the average 1–3 Hz power recorded in each hemisphere during SWS with the contralateral eye closure. Reported values are the means ±S.E. (LO/RC, n = 238 epochs; LC/RO, n = 441 epochs).
After the experiments were completed, the electrodes were removed and the whale was fully rehabilitated. Eight months later when we submitted this manuscript the animal was in good health swimming in a large salt water lake. The care and use of the whale was performed under the guidelines established by the Russian Ministry of Higher Education on the use of animals in biomedical research. The study protocol was approved by the Research Commission of the Severtsov Institute of Ecology and Evolution of the Russian Academy of Sciences.
The white whale tolerated the restriction during 4 recording days and did not show any struggling, and behavioral, respiratory or cardiac signs of pain or stress. Recording was started immediately after the implantation had been completed and the first episode of SWS was recorded within 3 h after the whale was returned to the pool. In this report we present the results of analysis of the first 2 recording days when EEG recording was best while a full analysis of these data will be given later. During the first 2 days the white whale spent 43% of the recording time in SWS. Eighty-three percent of SWS in this whale was composed of episodes with highly pronounced EEG asymmetry (Fig. 1). Sixty-eight percent of total SWS time consisted of unambiguous unihemispheric low or high amplitude SWS (Table 1). The remaining time was almost equally occupied by episodes of highly asymmetrical but unambiguous bilateral SWS (high amplitude SWS in one hemisphere and low amplitude SWS in the other) and bilateral low amplitude SWS. We scored only several epochs (on average 60 s per day) as bilateral high amplitude SWS confirming the previous data that bilateral high amplitude SWS is not characteristic of Cetaceans under normal conditions [8,9,11,12]. It is important to emphasize that in most of the epochs scored as bilateral SWS the difference in the amplitude and in the frequency of EEG between the two hemispheres was also easily recognized visually. EEG spectral analysis revealed that EEG power density in the range of 1–3 Hz differed significantly in the two hemispheres in the whale and almost never approached high values in each hemisphere at the same time (Fig. 1A). Therefore, the white whale is the fifth representative of Cetaceans studied electrophysiologically and in all five studied species sleep is typically composed of unihemispheric and highly asymmetric SWS.
Table 1.
Composition of slow sleep in a white whale (percentage of the total slow wave sleep time recorded over the 48-h period)
| Type of slow wave sleep | Percentage |
|---|---|
| Unihemispheric low amplitude | 46.8 |
| Unihemispheric high amplitude | 21.3 |
| Asymmetrical bilateral | 14.7 |
| Bilateral low amplitude | 17.0 |
| Bilateral high amplitude (delta) | 0.2 |
In this study we documented for the first time a relationship between the EEG in the two hemispheres and the state of the eyes in a sleeping Cetacean. Bilateral EEG desynchronization (waking) in this whale was highly related to bilateral eye opening (80% of all epochs in which both eyes were open). Episodes of bilateral eye closure were rare in this whale and occupied less than 1.6% of the observation time. Surprisingly, 49% of bilateral eye closure occurred during episodes of bilateral EEG desynchronization. The remaining episodes of bilateral eye closure were recorded during low amplitude unilateral (32%) or bilateral SWS (16%) and rarely during high amplitude SWS (only 3% of all bilateral eye closure episodes). This data indicates that bilateral eye closure in this white whale was not related to the deepest SWS, the opposite of what is characteristic of all terrestrial mammals [17] and birds [15,16].
When the whale was awake (bilateral desynchronized EEG) there was no difference between the states of the two eyes (χ2-test; df=2; P > 0.1; Fig. 2, lower panel). During unihemispheric or asymmetrical SWS (Fig. 2, top and middle panels) the eye contralateral to the sleeping more deeply hemisphere was usually closed (right eye, 52% of the total sleep time in the contralateral hemisphere during 2 consecutive days; left eye, 40%) or in an intermediate state (31 and 46%, respectively) and the ipsilateral eye was typically open (89 and 80%). The difference between the state of the two eyes during asymmetrical or unihemispheric SWS was highly significant (χ2-test; df=2; P ≪ 0.001). The data holds important implications of our data for interpretation of results of visual observations on Cetacean behavior [2,3]. Prolonged episodes of unilateral eye closure most likely include episodes of unihemispheric SWS in slowly swimming Cetaceans but the behavioral state (sleep or waking) cannot be accurately predicted on the basis of the state of the eyelids alone.
Fig. 2.
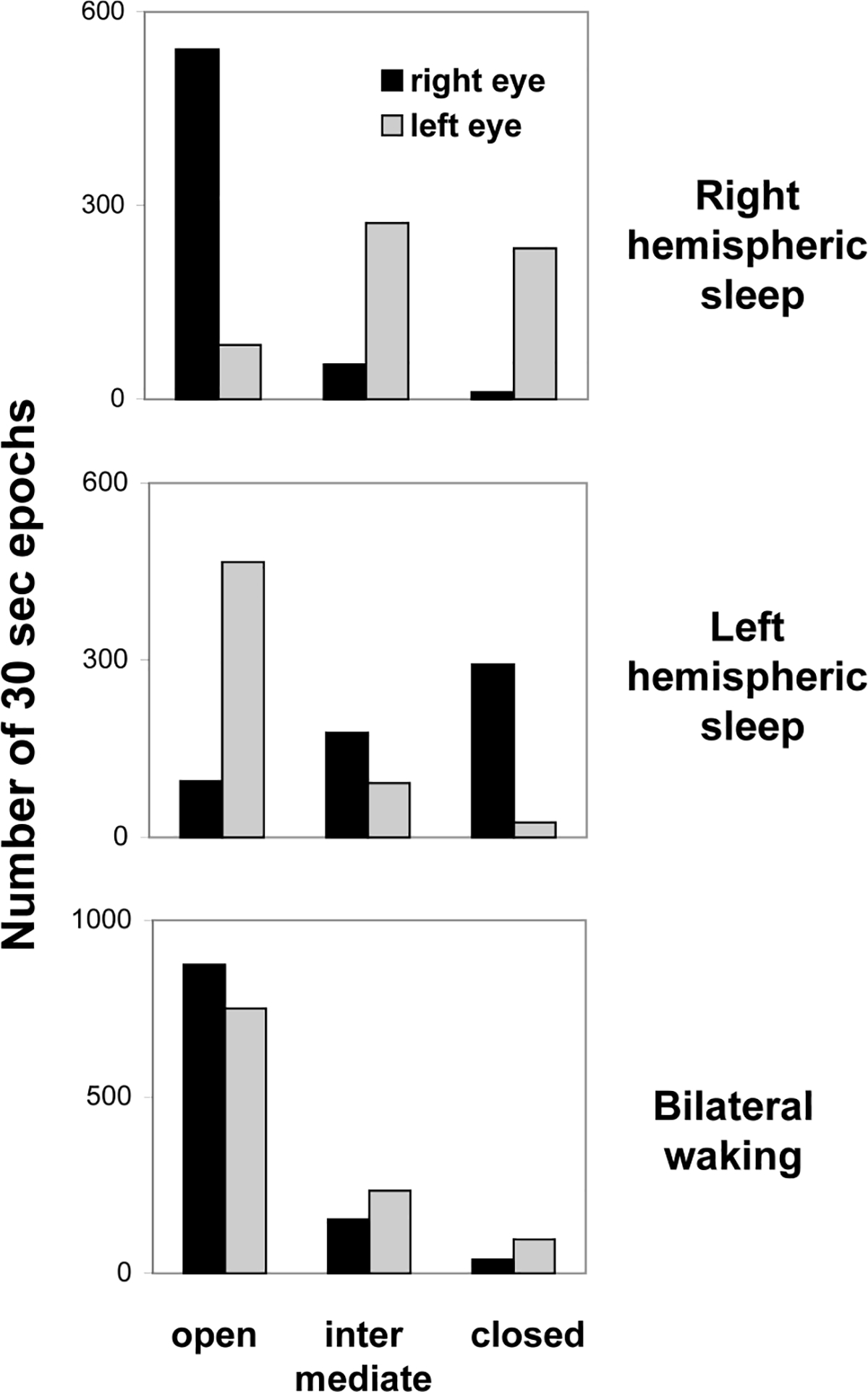
The state of the eyes during waking and sleep in a white whale. The EEG stage in each hemisphere was scored visually in 30 s. The state of the eyes was sampled in 5-s epochs and then extrapolated for consecutive 30-s epochs. Right and left hemispheric sleep represents unihemispheric SWS or asymmetrical bilateral SWS with a higher voltage EEG in the corresponding hemisphere. Reported values are the numbers of 30-s epochs with a given state of the eyes documented over 2 consecutive days.
The spectral analysis of the EEG from two hemispheres simultaneously with sampling of eye state in 5-s epochs performed during a 3-h sleep confirmed the results of analysis in 30-s epochs. Fig. 1 illustrates that when one eye was closed, the EEG spectral power in the range of 1–3 Hz (5-s epochs) in the contralateral hemisphere was significantly higher than that in the ipsilateral hemisphere (Fig. 1C). This difference in the spectral power between the two hemispheres in this beluga was much greater (on average more than 1000%) than that described during episodes of sleep with unilateral eye closure in birds [15].
In spite of the evident overall relationship between the sleeping hemisphere and the position of the eyelids observed in this white whale, transient changes in the state of one eye were not always accompanied by immediate changes in the EEG (Fig. 1B). We analyzed how a change in the state of each eye (full opening, full closing, part opening and part closing) related to parallel changes in the EEG in the two hemispheres. For data related to eye state (when both eyes were clearly visible), we used visually scored EEG in 30-s epochs (a total of 11 h of sleep) and EEG spectral analysis in 5-s epochs (the 3-h period of sleep presented in Fig. 1A). In both cases we found no statistically significant association between changes in the state of the eyelids and the EEG (visual scoring and spectral analysis) in these 5 and 30-s time intervals. For instance, in only 55% of cases was a full opening of the eye contralateral to the sleeping hemisphere accompanied by at least a 50% decrease in the EEG spectral power in the contralateral hemisphere, meaning that eye opening and arousal were not significantly correlated (29 transitions between two consecutive 5-s epochs; P > 0.1; Wilcoxon matched-pairs test). We also documented a number of eye openings lasting between 31 and 70 s (15 episodes per 3 days) contralateral to the synchronized hemisphere (high amplitude SWS), which were not accompanied by changes in the EEG. Conversely during unihemispheric SWS we saw several eye closures lasting as long as 30 s contralateral to the desynchronized hemisphere. Thus, while EEG and the state of the eyes were highly correlated over long time periods in this whale, EEG and eye position could be independent over short time periods (about 1 min and shorter).
Though we cannot exclude that the confinement situation and artificial light might influence the total amount of SWS, composition of SWS, and the amount of time the whale spent with asymmetrical state of the two eyes, there is no doubt about the association between unihemispheric SWS and eye state in this whale. Earlier we suggested that unihemispheric sleep in dolphins allows them to sleep, swim and breathe at the same time [8,11,12]. This study of sleep in this white whale supports the idea that visual monitoring of the environment is also one of the functions of unihemispheric sleep in Cetaceans [2,3,10,11,13]. Moreover, the nature of the relationship between the EEG and unilateral eye opening we observed in this whale (specifically we refer to overall relationship over long time periods in this whale and relative independency over short time periods) suggests that unihemispheric sleep is a state which allows Cetaceans to sleep with one eye open, rather than unilateral eye opening leading to the initiation of unihemispheric sleep. It is also well known that some Cetaceans (river dolphins) exhibit unihemispheric SWS [9] even though they live in very muddy water and have very poor vision. In addition, observations in an aquarium revealed that practically blind Indus dolphins were constantly swimming on the side touching the floor with their flippers [14]. Therefore, we hypothesize that unihemispheric sleep may serve dolphins to perform also other (non-visual) sensory monitoring of their habitat such as auditory and, in some species, tactile integration.
To summarize, the present knowledge on unihemispheric SWS in Cetaceans and Pinnipeds [1,4,8,16] and episodes of EEG asymmetry in birds [15,16] suggests the following: (1) Unihemispheric sleep is an active state, rather than a result of asymmetrical sensory activation in the visual system; (2) Unihemispheric sleep can assist in performing several functions during sleep, including sensory monitoring of the environment (first of all visual; Cetaceans, Pinnipeds and birds), maintaining of movement (swimming in marine mammals and probably flight in some birds) and voluntary control of respiratory functions in Cetaceans.
Acknowledgements
The study was supported by Utrish Dolphinarium Ltd. and the Medical Research Service of the Veterans Administration, USPHS grant NS32819. The authors thank E. Rozanova for veterinary support and two anonymous reviewers whose comments improved the final version of the manuscript.
References
- [1].Castellini MA. Sleep in aquatic mammals. In: Carley D, Radulovacki M, editors. Animal Models of Sleep Related Breathing Disorder. Lung Biology in Health and Disease. New York: Marcel Dekker, 2001, in press. [Google Scholar]
- [2].Goley PG. Behavioral aspects of sleep in pacific white-sided dolphins (Lagenorhynchus obliquidens, Gill 1866). Mar Mamm Sci 1999;15:1054–64. [Google Scholar]
- [3].Lilly JC. Animals in aquatic environments: adaptation of mammals to the ocean. In: Dill DB, Adolph EF, Wilber CG, editors. Handbook of Physiology—Adaptation to the Environment. Washington, DC: American Physiology Society, 1964:741–7. [Google Scholar]
- [4].Lyamin OI, Chetyrbok IS. Unilateral EEG activation during sleep in the cape fur seal, Arctocephalus pusillus. Neurosci Lett 1992;143:263–6. [DOI] [PubMed] [Google Scholar]
- [5].Lyamin OI, Manger PR, Mukhametov LM, Siegel JM, Shpak OV. Rest and activity states in a gray whale. J Sleep Res 2000;9:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lyamin OI, Shpak OV, Nazarenko EA, Mukhametov LM. Behavioral signs of paradoxical sleep in the beluga whale. J Sleep Res 1998;7(Suppl. 2):166. [Google Scholar]
- [7].McCormick JG. Relationship of sleep, respiration, and anesthesia in the porpoise: a preliminary report. Proc Natl Acad Sci USA 1969;62:697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mukhametov LM. Sleep in marine mammals. Exp Brain Res 1984;8:227–38. [Google Scholar]
- [9].Mukhametov LM. Unihemispheric slow-wave sleep in the Amazonian dolphin, Inia geoffrensis. Neurosci Lett 1987;79:128–32. [DOI] [PubMed] [Google Scholar]
- [10].Mukhametov LM, Lyamin OI. The Black Sea bottlenose dolphin: the conditions of rest and activity. In: Sokolov VE, Romanenko EV, editors. The Black See Bottlenose Dolphin. Moscow: Nauka, 1997:650–68 (in Russian). [Google Scholar]
- [11].Mukhametov LM, Oleksenko AI, Polyakova IG. The Black see bottlenose dolphin: the structure of sleep. In: Sokolov VE, Romanenko EV, editors. The Black See Bottlenose Dolphin. Moscow: Nauka, 1997:492–512 (in Russian). [Google Scholar]
- [12].Mukhametov LM, Supin AY, Polyakova IG. Interhemispheric asymmetry of the electroencephalographic sleep patterns in dolphins. Brain Res 1977;134:581–4. [DOI] [PubMed] [Google Scholar]
- [13].Oleksenko AI, Chetyrbok IS, Polyakova IG, Mukhametov LM. Rest and activity states in Amazonian dolphins. In: Sokolov VE, Romanenko EV, editors. The Amazonian Dolphin. Moscow: Nauka, 1996:257–66. [Google Scholar]
- [14].Pilleri G The blind Indus dolphin, Platanista indi. Endeavour 1979;3:45–56. [Google Scholar]
- [15].Rattenborg NC, Lima SL, Amlaner CJ. Facultative control of avian unihemispheric sleep under the risk of predation. Behav Brain Res 1999;105:163–72. [DOI] [PubMed] [Google Scholar]
- [16].Rattenborg NC, Amlaner CJ, Lima SL. Behavioral, neurological and evolutionary perspectives on unihemispheric sleep. Neurosci Biobehav Rev 2000;24:817–42. [DOI] [PubMed] [Google Scholar]
- [17].Zepelin A Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine, 3rd ed Philadelphia: Saunders, 2000:82–92. [Google Scholar]


