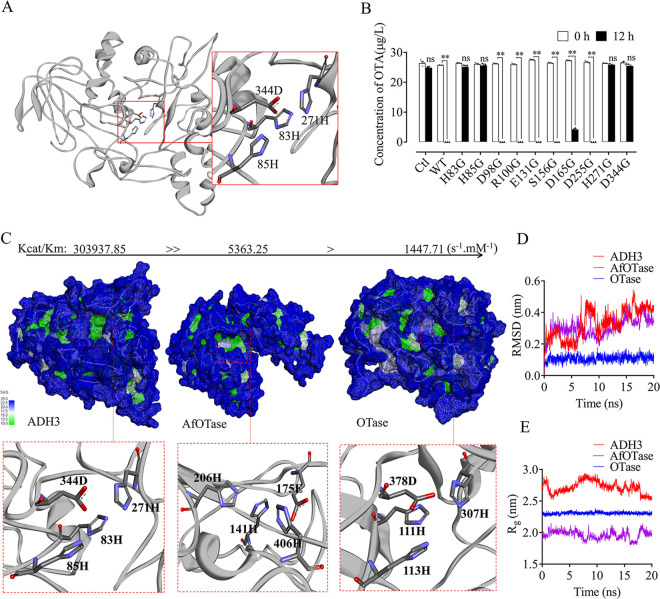
FIG 7.
Comparison of the catalytic center structures of different OTA degrading enzymes. (A, B) Activity analysis linked point mutation experiments demonstrated that four amino acid residues (83H, 85H, 271H, and 344D) that constitute the quaternary catalytic center are critical to OTA degradation activity of ADH3. (C) Solvent accessibility (SAS) results indicated that the catalytic site of ADH3 was more hydrophobic than that in Otase. (D, E) Molecular dynamic analysis of the four residues of quaternary catalytic site of AHD3, possessed the highest root mean squared deviation (RMSD, D) and radius of gyration (Rg, E).