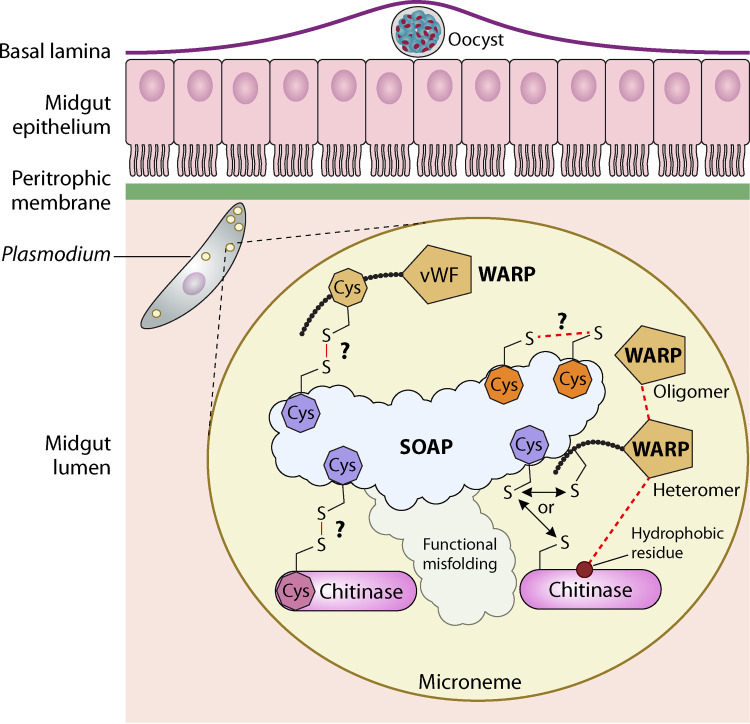
FIG 6.
Schematic representation of the mosquito midgut invasion process where Plasmodium-secreted SOAP acts as a scaffold protein. SOAP is predicted to be unstructured/disordered for Plasmodium species. The multiple cysteine residues (dark-orange octahedral structure) present in the structure might be involved in forming intramolecular disulfide linkages. However, the conserved cysteine residue (purple octahedral structure; shown three times for better representation) in Pf/PgSOAP might be present as a free residue in the less-conserved region separating two cysteine-rich domains. The functional misfolding of the SOAP protein may prevent the nonspecific interactions of the cysteines by hiding the key elements inside nonreactive grooves and thus enable interaction with only native species-specific binding partners (chitinase and WARP) through covalent intermolecular linkages. The WARP protein forms oligomers and also heteromers by enabling its interaction with hydrophobic patches present on the chitinase surface (shown with the forward slash). The hetero-multimeric WARP-chitinase protein complex then might interact with the SOAP protein through the conserved cysteine of WARP or surface-exposed cysteine residue present in the chitinase protein for intermolecular disulfide linkages. The details about the biogenesis and localization of the hetero-multimeric complex remains to be further elucidated; this is where the biogenesis may take place as soon as the proteins are secreted from the membrane-bound microneme from the apical end of the parasite. Understanding these details will uncover fundamental aspects of Plasmodium invasion-related organelle function, with direct relevance to understanding the role of micronemal biogenesis and transport in mosquito midgut invasion. (The figure was created with BioRender.)