FIGURE 1.
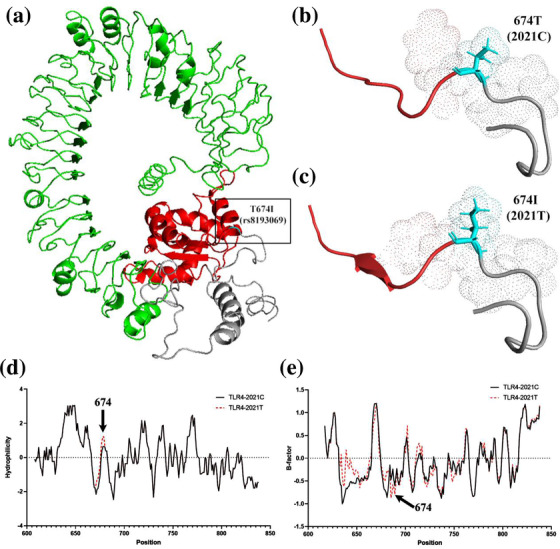
Effects of rs8193069 on TLR4 protein structure and function. (a) The location of amino acid variation corresponding to rs8193069 on theoretical three‐dimensional model of TLR4 protein. The variation is marked with cyan. The TIR domain (marked with red) is located in the membrane and contains amino acids 674–814. The extracellular part of TLR4 is indicated with green. (b), (c) The predicted three‐dimensional structure of TLR4 when the rs8193069 was C and T, respectively. (d) Effects of rs8193069 on hydrophilicity of TLR4. It shows the region at amino acid 603–841. The mutation of rs8193069 from C to T results in increased hydrophilicity values of the nearby amino acids. Higher positive values indicate stronger hydrophobicity and lower negative values indicate stronger hydrophilicity. (e) Effect of rs8193069 on flexibility of TLR4. It shows the region at amino acid 603–841. The mutation of rs8193069 from C to T results in decreased B‐factor values of the nearby amino acids, which indicate the reduced flexibility
