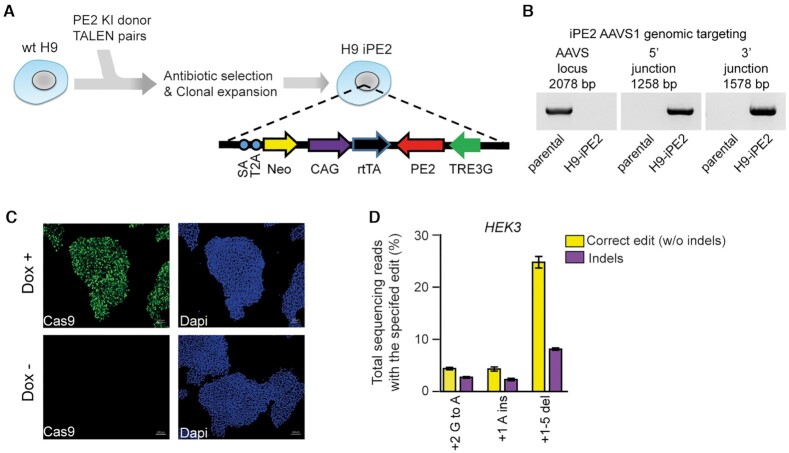
Figure 1.
Generation and characterization of H9-iPE2. (A) Schematic diagram of the strategy for TALEN-mediated targeting of the AAVS1 locus to generate H9-iPE2 cells, in which PE2 expression is induced by dox. The AAVS1 donor vector contains a cassette in which PE2 expression is under the control of the dox-inducible TRE3G promoter. SA, splice acceptor; 2A, self-cleaving 2A peptide; Neo, neomycin resistance gene; rtTA, dox-controlled reverse transcriptional activator; CAG, cytomegalovirus early enhancer/chicken β actin promoter. (B) PCR-based confirmation that the iPE2 construct was targeted to the AAVS1 locus. A primer pair that flanks the AAVS1 knock-in site only amplifies a product in wild-type cells; lack of a product indicates that the H9-iPE2 clone used in this study is homozygous (left panel). Junction PCR confirmed on-target integration of the cassette into the AAVS1 locus (center and right panels). (C) Immunostaining of H9-iPE2 cells before and after 48 h of dox treatment with an anti-Cas9 antibody (green). Nuclei were stained with DAPI (blue). (D) Editing efficiency in H9-iPE2 cells. H9-iPE2 cells maintained in the presence of dox were electroporated with plasmids encoding a pegRNA and a nicking sgRNA (for PE3 system). The editing efficiency is indicated as the percentage of total sequencing reads that contain the intended edit and do not contain indels. Mean ± s.d. of n = 3 independent biological replicates.