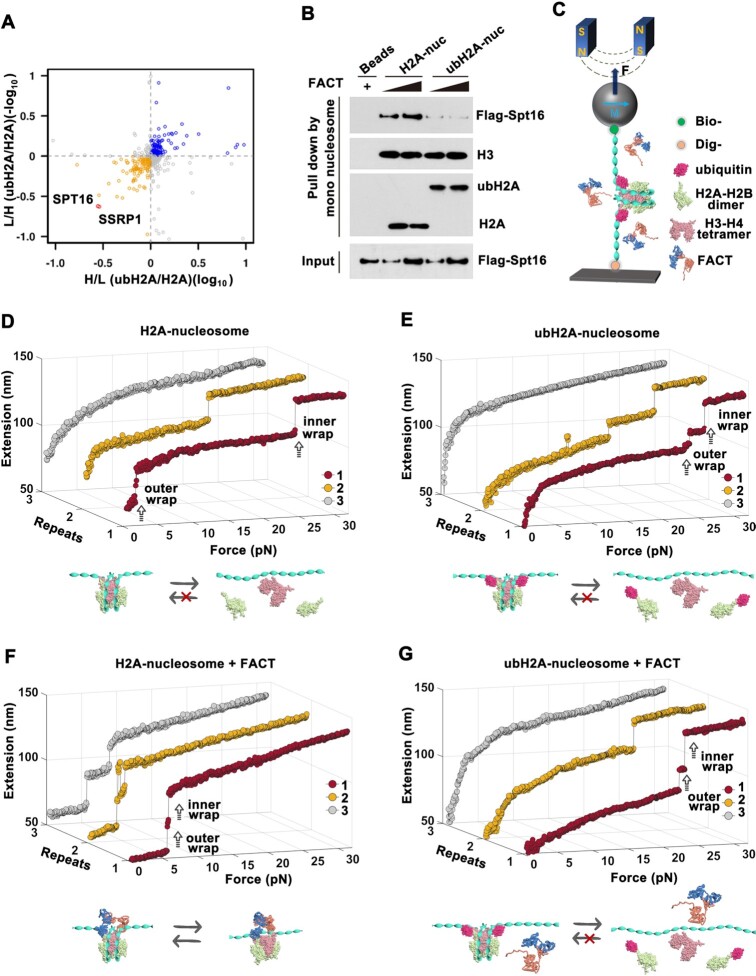
Figure 1.
ubH2A inhibits the binding of FACT on nucleosome and blocks the remodeling function of FACT. (A) Quantitative SILAC-based mass spectrometry analysis of mono-nucleosome pull-down assays. H2A and ubH2A nucleosomes were incubated with heavy or light nuclear extracts of HEK293T cells (n = 4 biologically independent experiments). (B) Mono-nucleosome pull-down assay of H2A and ubH2A mono-nucleosome incubated with the purified FACT. The immunoblots are representative of three independent experiments. (C) A schematic representation of single-molecule stretching experiment (not to scale): a single H2A or ubH2A mono-nucleosome is tethered between a coverslip and a superparamagnetic bead. (D–G) Repeated stretching measurements of H2A and ubH2A mono-nucleosome without (D, E) and with (F, G) FACT. The cartoons below represent the relative dynamic process of the nucleosome, respectively. In each stretching cycle, the force is increased up to 32 pN at a loading rate of 0.1 pN/s.