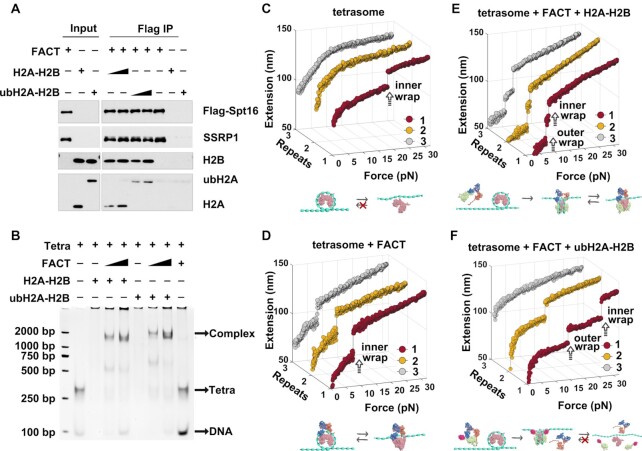
Figure 3.
FACT can deposit ubH2A–H2B dimer onto tetrasome to form intact nucleosome. (A) Immunoprecipitation assays of the recombinant FACT with Flag-SPT16 and SSRP1 incubated with recombinant H2A–H2B or ubH2A–H2B dimer. The results showed that ubH2A does not affect the binding property of FACT on H2A–H2B dimer. (B) The gel electrophoresis analysis of the binding of FACT-H2A–H2B or FACT-ubH2A–H2B with (H3–ṇH4)2 tetrasome, which showed that FACT can promote the binding of H2A–H2B to tetrasomes to form a ternary complex with FACT-H2A–H2B and (H3–ṇH4)2 tetrasome, and the ubH2A does not affect the binding of FACT-H2A–H2B on (H3–ṇH4)2 tetrasome. (C–F) The repeated stretching measurements of the mono-tetrasome alone (C), with FACT alone (D), with FACT and H2A–H2B dimer (E), and with FACT and ubH2A–H2B dimer (F), respectively. The cartoons below represent the relative dynamic process of the tetrasome or nucleosome, respectively.