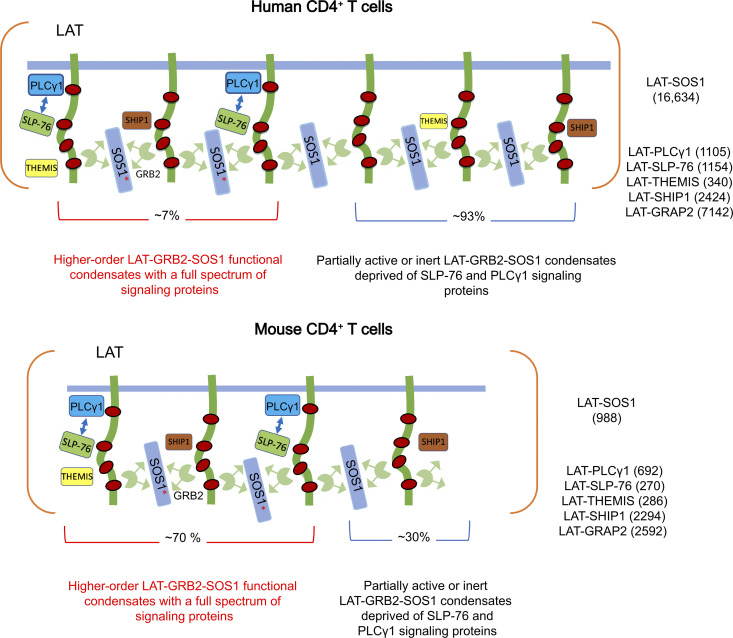
Figure 7.
Model summarizing the composition and stoichiometry of LAT condensates in human and mouse primary CD4+ T cells. Model of the LAT-GRB2-SOS1–based condensates forming in human (top) and mouse (bottom) CD4+ T cells. The intrinsically disordered cytoplasmic tail of LAT is phosphorylated by ZAP-70 on at least three tyrosine residues (red dots) in response to TCR engagement, allowing interaction with a constellation of cytoplasmic proteins containing SH2 domains. These proteins also contain SH3 domains and proline-rich motifs, allowing them to establish multivalent interactions and enabling their assembly into scaffolds, and concomitant protein phase separation (Su et al., 2016). The client proteins recruited by LAT-GRB2-SOS1–based scaffold comprise SLP-76, THEMIS, SHIP-1, PIK3 p85a, GRAP2, and PLCγ1. They interact directly (PLCγ1 and GRAP2) or indirectly (THEMIS, SHIP-1, PIK3 p85a) with tyrosine phosphorylated LAT molecules. The maximal numbers of copies per T cell of LAT-SOS1, LAT-PLCγ1, LAT-SLP76, LAT-GRAP2, LAT-THEMIS, and LAT-SHIP-1 interactions reached 30 s after TCR engagement are specified in the right part of the human and mouse panels. These numbers likely correspond to a single conglomerate of individual LAT condensates (containing each a few hundreds of LAT molecules; McAffee et al., 2021 Preprint) that concatenated during the affinity purification process [see Discussion]). Approximately 70% and 7% of the LAT-GRB2-SOS1 scaffold of mouse and human CD4+ T cells, respectively, are occupied by SLP-76 and PLCγ1 signaling clients, giving rise to comparable numbers of higher-order LAT-GRB2-SOS1-SLP-76-PLCγ1 signaling condensates capable of delivering full-fledged signals (see Results). SOS1 recruitment to the plasma membrane depends on its GRB2-dependent binding to phosphorylated LAT. This allows the autoinhibitory domains of SOS1 to interact with negatively charged membrane lipids, leading to structural rearrangements and subsequent release of autoinhibition. However, SOS1 full activity is contingent on prior activation of the the RAS exchange factor RAS-GRPP1 by the second messenger molecules diacylglycerol and calcium generated by active PLCγ1 molecules. It results in the production of RAS-GTP molecules that bind to an allosteric pocket on SOS1, increasing its catalytic activity up to 80-fold and allowing it to catalyzes nucleotide exchange on RAS molecules (Jun et al., 2013). Considering that RAS-GRP1 activity depends on PLCγ1 activity, fully active SOS1 molecules (red asterisk) are likely located within the higher-order LAT-GRB2-SOS1-SLP-76-PLCγ1 signaling condensates. In contrast, LAT-GRB2-THEMIS and LAT-GRB2-SHIP-1 ternary interactions do not rely on multivalent weak cooperative interactions as LAT, GRAP2, SLP-76, and PLCγ1 do, and are likely evenly distributed among the LAT-GRB2-SOS1 scaffold. The excess of LAT-GRAP2 interactions over LAT-SLP76 interactions in both mouse and human CD4+ T cells is likely due to the competition that exists between preformed GRAP2-SLP-76 binary complexes and the larger pool of free GRAP2 adaptors for binding to phosphorylated LAT molecules (Mori et al., 2021). For the sake of simplicity, RAS-GRP1, the GRAP2 adaptor bridging LAT and SLP-76 molecules, and the GRB2 adaptor bridging LAT and either THEMIS or SHIP-1 molecules are not represented. Some client proteins such as PLCγ1 can also contribute to phase separation (Zeng et al., 2021). SLP-76 has also been reported to contribute to oligomerization by binding to the adaptor molecule FYN-binding protein 1 (FYB; also known as ADAP; Coussens et al., 2013). However, we failed to detect FYB in the SLP-76 and LAT interactomes of both human and mouse primary T cells as well as VAMP7 in the LAT interactome (Data S1; Mori et al., 2021).