Abstract
Background
We evaluated our SARS-CoV-2 prefusion spike recombinant protein vaccine (CoV2 preS dTM) with different adjuvants, unadjuvanted, and in a one-injection and two-injection dosing schedule in a previous phase 1–2 study. Based on interim results from that study, we selected a two-injection schedule and the AS03 adjuvant for further clinical development. However, lower than expected antibody responses, particularly in older adults, and higher than expected reactogenicity after the second vaccination were observed. In the current study, we evaluated the safety and immunogenicity of an optimised formulation of CoV2 preS dTM adjuvanted with AS03 to inform progression to phase 3 clinical trial.
Methods
This phase 2, randomised, parallel-group, dose-ranging study was done in adults (≥18 years old), including those with pre-existing medical conditions, those who were immunocompromised (except those with recent organ transplant or chemotherapy) and those with a potentially increased risk for severe COVID-19, at 20 clinical research centres in the USA and Honduras. Women who were pregnant or lactating or, for those of childbearing potential, not using an effective method of contraception or abstinence, and those who had received a COVID-19 vaccine, were excluded. Participants were randomly assigned (1:1:1) using an interactive response technology system, with stratification by age (18–59 years and ≥60 years), rapid serodiagnostic test result (positive or negative), and high-risk medical conditions (yes or no), to receive two injections (day 1 and day 22) of 5 7mu;g (low dose), 10 7mu;g (medium dose), or 15 7mu;g (high dose) CoV2 preS dTM antigen with fixed AS03 content. All participants and outcome assessors were masked to group assignment; unmasked study staff involved in vaccine preparation were not involved in safety outcome assessments. All laboratory staff performing the assays were masked to treatment. The primary safety objective was to describe the safety profile in all participants, for each candidate vaccine formulation. Safety endpoints were evaluated for all randomised participants who received at least one dose of the study vaccine (safety analysis set), and are presented here for the interim study period (up to day 43). The primary immunogenicity objective was to describe the neutralising antibody titres to the D614G variant 14 days after the second vaccination (day 36) in participants who were SARS-CoV-2 naive who received both injections, provided samples at day 1 and day 36, did not have protocol deviations, and did not receive an authorised COVID-19 vaccine before day 36. Neutralising antibodies were measured using a pseudovirus neutralisation assay and are presented here up to 14 days after the second dose. As a secondary immunogenicity objective, we assessed neutralising antibodies in non-naive participants. This trial is registered with ClinicalTrials.gov (NCT04762680) and is closed to new participants for the cohort reported here.
Findings
Of 722 participants enrolled and randomly assigned between Feb 24, 2021, and March 8, 2021, 721 received at least one injection (low dose=240, medium dose=239, and high dose=242). The proportion of participants reporting at least one solicited adverse reaction (injection site or systemic) in the first 7 days after any vaccination was similar between treatment groups (217 [91%] of 238 in the low-dose group, 213 [90%] of 237 in the medium-dose group, and 218 [91%] of 239 in the high-dose group); these adverse reactions were transient, were mostly mild to moderate in intensity, and occurred at a higher frequency and intensity after the second vaccination. Four participants reported immediate unsolicited adverse events; two (one each in the low-dose group and medium-dose group) were considered by the investigators to be vaccine related and two (one each in the low-dose and high-dose groups) were considered unrelated. Five participants reported seven vaccine-related medically attended adverse events (two in the low-dose group, one in the medium-dose group, and four in the high-dose group). No vaccine-related serious adverse events and no adverse events of special interest were reported. Among participants naive to SARS-CoV-2 at day 36, 158 (98%) of 162 in the low-dose group, 166 (99%) of 168 in the medium-dose group, and 163 (98%) of 166 in the high-dose group had at least a two-fold increase in neutralising antibody titres to the D614G variant from baseline. Neutralising antibody geometric mean titres (GMTs) at day 36 for participants who were naive were 2189 (95% CI 1744–2746) for the low-dose group, 2269 (1792–2873) for the medium-dose group, and 2895 (2294–3654) for the high-dose group. GMT ratios (day 36: day 1) were 107 (95% CI 85–135) in the low-dose group, 110 (87–140) in the medium-dose group, and 141 (111–179) in the high-dose group. Neutralising antibody titres in non-naive adults 21 days after one injection tended to be higher than titres after two injections in adults who were naive, with GMTs 21 days after one injection for participants who were non-naive being 3143 (95% CI 836–11 815) in the low-dose group, 2338 (593–9226) in the medium-dose group, and 7069 (1361–36 725) in the high-dose group.
Interpretation
Two injections of CoV2 preS dTM-AS03 showed acceptable safety and reactogenicity, and robust immunogenicity in adults who were SARS-CoV-2 naive and non-naive. These results supported progression to phase 3 evaluation of the 10 7mu;g antigen dose for primary vaccination and a 5 7mu;g antigen dose for booster vaccination.
Funding
Sanofi Pasteur and Biomedical Advanced Research and Development Authority.
Introduction
COVID-19 has inflicted unprecedented morbidity and mortality worldwide and continues to devastate global health and economies more than 2 years since its emergence.1, 2 Extraordinary efforts in the development, manufacturing, and distribution of COVID-19 vaccines have led to several vaccines being granted emergency-use designation3 or full approval. Continued efforts to develop vaccines remain necessary to meet global demand, to offer alternative vaccine choices with benefit–risk profiles optimised for diverse populations, and to provide broader protection against emerging variants.
Research in context.
Evidence before this study
We searched PubMed from database inception up to Sept 27, 2021, with no language restrictions, for studies reporting the safety and immunogenicity of adjuvanted recombinant protein vaccine candidates against SARS-CoV-2 using the search terms “vaccine”, “clinical trial”, “SARS-CoV-2”, “recombinant AND protein”, and “adjuvant”. Among published trials, one phase 1 study showed acceptable safety and immunogenicity of a subunit vaccine containing MF59-adjuvanted, molecular clamp-stabilised recombinant spike protein (NCT04495933), and a phase 1–2 safety and immunogenicity trial (NCT04368988) and a phase 2 efficacy trial (NCT04533399) showed that the NVX-CoV2373 nanoparticle vaccine (containing recombinant spike protein adjuvanted with matrix-M1 adjuvant) had an acceptable safety profile and was effective against laboratory-confirmed symptomatic COVID-19, including in patients who were HIV positive and against cases caused by the beta (B.1.351) variant. Although not retrieved in our search, a recently published phase 3 trial in 14 039 participants reported a vaccine efficacy of 89·7% against SARS-CoV-2 infection with the NVX-CoV2373 vaccine and high efficacy against the alpha (B.1.1.7) variant (EudraCT number 2020-004123-16). Another protein-subunit vaccine candidate containing a stabilised trimeric form of the spike protein combined with either AS03 or CpG with alum adjuvants has also shown acceptable safety and immunogenicity in phase 1 evaluation (NCT04405908). Preliminary data for other SARS-CoV-2 vaccine candidates (including a virus-like particle vaccine manufactured in plants) have additionally shown evidence of acceptable safety and promising immunogenicity profiles when adjuvanted with the AS03 adjuvant system. In a Phase 1–2 study (NCT04537208), we previously evaluated the safety and immunogenicity of our recombinant SARS-CoV-2 protein candidate vaccine, CoV2 preS dTM, combined with the AS03 adjuvant system or AF03 adjuvant, at two different antigen doses in healthy adults (aged 18 years or older). Interim data from that study enabled the selection of a two-injection schedule and the AS03 adjuvant for further clinical development, although lower-than-expected antibody responses, particularly in older adults, and higher-than-expected reactogenicity were observed. These were hypothesised to be caused by the formulations tested having lower-than-planned antigen doses and higher-than-anticipated host-cell protein content. Therefore, there was a need to optimise the vaccine formulation.
Added value of this study
The current phase 2 study showed an acceptable safety and reactogenicity profile, and favourable immune responses, of two injections of optimised CoV2 preS dTM formulations adjuvanted with AS03, at three different antigen doses (5 7mu;g, 10 7mu;g, or 15 7mu;g CoV2 preS dTM antigen), in adults who were SARS-CoV-2 naive and non-naive, including those in high-risk groups (ie, aged ≥60 years or with pre-existing medical conditions, or both). Our findings supported progression of the 10 7mu;g dose formulation to phase 3 efficacy evaluation (NCT04904549). Furthermore, given the high neutralising antibody titres and acceptable safety profile after a single vaccine dose observed in participants with evidence of previous SARS-CoV-2 infection, we are evaluating the lower antigen dose (5 7mu;g) for use as a booster vaccine (NCT04762680).
Implications of all the available evidence
These data support progression of the CoV2 preS dTM with AS03 adjuvant vaccine candidate to phase 3 clinical evaluation (NCT04904549), representing an important step in the continued efforts to expand available options for the global supply of safe and effective SARS-CoV-2 vaccines. This adjuvanted recombinant protein vaccine, using a well established vaccine-manufacturing platform with favourable cold-chain requirements (distribution at 2–8°C), offers an alternative to currently approved vaccines.
Sanofi Pasteur, in collaboration with GlaxoSmithKline, developed an adjuvanted SARS-CoV-2 recombinant-protein vaccine using a baculovirus expression-vector system to express a stabilised SARS-CoV-2 prefusion spike (S) protein (CoV2 preS dTM).4 The use of an adjuvanted vaccine formulation offers advantages of dose sparing and greater breadth of protection.5 In a phase 1–2 study, the safety and immunogenicity of the CoV2 preS dTM candidate vaccine adjuvanted with AS03 (CoV2 preS dTM-AS03; GlaxoSmithKline) or AF03 (Sanofi Pasteur) at two antigen doses (5 7mu;g for the low dose and 15 7mu;g for the high dose) were evaluated in healthy adults aged 18 years or older.4 Interim data from that study enabled selection of a two-injection schedule and the AS03 adjuvant for further clinical development. However, lower-than-expected antibody responses, particularly in older adults (≥60 years), and higher-than-expected reactogenicity after the second vaccination were observed. The phase 1–2 clinical trial formulations tested had lower-than-anticipated antigen concentrations (1·3 7mu;g for the low dose and 2·6 7mu;g for the high dose), which we hypothesised contributed to the reduced antibody response. The increased reactogenicity was hypothesised to be caused by a higher-than-anticipated host-cell protein content in the vaccine formulations.4 In this study, we aimed to evaluate the safety, reactogenicity, and immunogenicity of three optimised formulations of CoV2 preS dTM-AS03.
Methods
Study design and participants
This is an ongoing phase 2, randomised, modified double-blind, parallel group, dose-ranging study, done in 20 clinical research centres in the USA and Honduras, with a planned duration of approximately 13 months. Here, we present interim safety and reactogenicity data up to study day 43, 3 weeks after the second vaccination, and immunogenicity data up to study day 36.
Adults aged 18 years and older were eligible for inclusion in the study. Women who were pregnant or lactating or, for those of childbearing potential, not using an effective method of contraception or abstinence from at least 4 weeks before the first dose until at least 12 weeks after the second dose, and those who had received a COVID-19 vaccine, were excluded. To allow evaluation of vaccine performance in high-risk groups, individuals with pre-existing medical conditions, those who were immunocompromised (except those who had received solid-organ or bone marrow transplant in the past 180 days or chemotherapy in the past 90 days), and those with a potentially increased risk for severe COVID-196 were eligible for participation in the study. The inclusion and exclusion criteria, and the list of medical conditions considered to be associated with an increased risk of severe COVID-19, are described in full in the appendix (p 4).
The study was done in compliance with the International Conference on Harmonisation guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The protocol and amendments were approved by applicable independent ethics committees and institutional review boards and the regulatory agencies, as per local regulations. Written informed consent was obtained from participants before any study procedures were done.
Randomisation and masking
A randomisation list was generated by an independent group with planned randomisation and a built-in interactive response technology system before the start of enrolment. The randomisation had three stratification factors, comprising age group (18–59 years and ≥60 years), baseline SARS-CoV-2 rapid serodiagnostic test positivity (positive or negative by COVID-19 immunoglobulin [Ig]G and IgM Rapid Test Cassette; Healgen Scientific, Houston, TX, USA), and high-risk medical conditions (yes or no). Within each stratum, participants were randomly assigned in a 1:1:1 ratio, with a block size of six, to receive two injections (on day 1 and day 22) of 5 μg (low dose), 10 μg (medium dose), or 15 μg (high dose) of CoV2 preS dTM antigen, with a fixed dose of AS03 adjuvant. A subset of participants who tested negative with the SARS-CoV-2 rapid serodiagnostic test were additionally stratified by age and study group and randomly assigned to provide samples for assessment of cell-mediated immunity and mucosal antibodies.
All participants and outcome assessors were masked to group assignment; unmasked study staff involved in vaccine preparation were not involved in safety outcome assessments. Additionally, all laboratory staff performing assays for the study were masked to treatment assignment.
Procedures
The recombinant protein antigen CoV2 preS dTM was produced using a Sanofi Pasteur proprietary insect-cell baculovirus expression-vector system, on the basis of an S-protein sequence from the Wuhan-Hu-1 (D614) reference strain, as previously described.4 The AS03 adjuvant system (GlaxoSmithKline Vaccines, Rixensart, Belgium) is an oil-in-water emulsion containing 11·86 mg α tocopherol and 10·69 mg squalene per 0·5 mL vaccine dose.4, 7 CoV2 preS dTM-AS03 vaccine formulations were presented in two separate vials, a multidose vial containing AS03 (sufficient for ten doses) and a single-dose vial containing one of the three antigen dose solutions. An equal volume of the adjuvant emulsion was added to the vial containing the antigen and mixed before injection. Vaccinations (0·5 mL per dose) were administered by qualified and trained study personnel by intramuscular injection into the deltoid region of the upper arm.
Blood samples and nasopharyngeal swabs were collected before each vaccination to establish whether participants had past or present SARS-CoV-2 infection (naive or non-naive). Participants were classified as naive or non-naive at day 1 and day 22 or day 1 or day 22 by assessment of blood samples using Elecsys electrochemiluminescence immunoassays for detection of anti-S antibodies (Elecsys Anti-SARS-CoV-2 S assay; Roche, Indianapolis, IN, USA) on study day 1 and for detection of anti-nucleocapsid antibodies (Elecsys Anti-SARS-CoV-2 N; Roche) on study days 1 and 22; and detection of SARS-CoV-2 nucleic acids in nasopharyngeal swabs using nucleic-acid amplification tests (NAAT; Abbott RealTime SARS-CoV-2 assay; Abbott Molecular, Des Plaines, IL, USA) on study days 1 and 22. Analyses were done according to the manufacturers' instructions. We defined participants as naive to SARS-CoV-2 on study days 1 and 22 if they tested negative for anti-S antibodies on study day 1 and for both anti-nucleocapsid antibodies and SARS-CoV-2 nucleic acids on days 1 and 22; we defined participants as non-naive if they tested positive on at least one of the three tests on study days 1, 22, or both (appendix p 5).
Blood samples will be collected from participants at all study visits up to day 387 for immunogenicity assessments; immunogenicity assessments at day 1, day 22, and day 36 are presented here. SARS-CoV-2 neutralising-antibody titres against the D614G variant and the beta (B.1.351) variant were measured with a pseudovirus neutralisation assay, using HIV-1 pseudovirions expressing the full-length S protein of the respective variant,8 at Monogram Biosciences LabCorp (South San Francisco, CA, USA). The pseudovirus neutralisation assay is described in detail in appendix p 6. Neutralising antibody titres were calculated as the reciprocal of the serum dilution resulting in 50% neutralisation. Binding antibody profiles were assessed by measuring SARS-CoV-2 anti-S protein IgG antibodies with an indirect ELISA (Nexelis, Laval, Canada), as described previously.4 The reference standard (006/GCN4/Std/01/2020) was prepared by pooling four 5 mL samples of COVID-19 convalescent serum (Quebec, Canada) from patients with no symptoms at least 14 days after infection. We established unitage in EU/mL, on the basis of the geometric mean of the half maximal effective concentration (EC50) from 69 valid standard curves, as an arbitrary concentration of 1142 EU/mL. Neutralising antibody and binding antibody responses to D614G were measured in all participants on day 1, day 22, and day 36. Neutralising antibody responses to the beta variant were measured at day 36.
Whole blood samples were stimulated ex vivo with SARS-CoV-2 S antigen (spike-GCN4; Nexelis), using the TruCulture system (Rules-Based Medicine, Austin, TX, USA) as described previously.4 A microsphere-based multiplex immunoassay (TruCulture OptiMAP assay; Rules-Based Medicine) was used to evaluate specific concentrations of interferon γ (IFNγ), tumour necrosis factor α (TNFα), interleukin (IL)-2, IL-4, IL-5, and IL-13 on validated cytokine-profiling panels. Reaction plates were analysed on a Luminex platform (Luminex Corporation, Austin, Texas, USA), and cytokine concentrations were calculated with adapted software (Rules-Based Medicine plate reader version 2.1.5.8; plate viewer version 5.1.1.2) using a standard curve for specific cytokine production at each timepoint.
Participants were provided a diary card to capture solicited and unsolicited adverse events for up to 21 days after the second injection; serious adverse events (SAEs), adverse events of special interest, and medically attended adverse events are being collected over the duration of the study. Adverse events were graded for intensity (from 1 [no interference with usual activities; ≥25 mm to ≤50 mm for injection site erythema and swelling; or ≥38·0°C to ≤38·4°C for fever] to 3 [severe and prevents usual activities; >100 mm for injection erythema and swelling; or ≥39·0°C for fever]) and were assessed by the investigator for seriousness and relatedness to the study vaccine. The Medical Dictionary for Regulatory Activities (MedDRA) system organ class and preferred term was recorded for unsolicited adverse events. Adverse events considered to be vaccine related were documented as an adverse reaction.
Outcomes
The primary safety objective was to describe the safety profile in all participants, for each candidate vaccine formulation. In this interim analysis, we describe primary safety endpoints up to day 43 (21 days after the second injection), which included unsolicited systemic adverse events within 30 min of each injection, solicited injection-site reactions (pain, erythema, and swelling) and solicited systemic reactions (fever, headache, malaise, myalgia, arthralgia, and chills) up to 7 days after each injection, unsolicited adverse events up to 21 days after the last injection, and medically attended adverse events, SAEs, and adverse events of special interest throughout the study. Adverse events of special interest included anaphylactic reactions, generalised convulsions, thrombocytopenia, and potential immune-mediated disorders.9 Secondary safety objectives (to be presented elsewhere) were laboratory-confirmed symptomatic COVID-19 and serologically confirmed SARS-CoV-2 infection.
The primary immunogenicity objective was to describe the neutralising antibody response to the D614G variant 14 days after the second vaccination (on day 36) in participants who were SARS-CoV-2 naive. Secondary immunogenicity objectives included assessing binding antibody responses in naive participants and binding antibody and neutralising antibody responses in participants who were non-naive. Antibody responses were described on the basis of geometric mean titres (GMTs) for neutralising antibodies or geometric mean concentrations (GMCs) for binding antibodies. We calculated GMT ratios (GMTRs) and GMC ratios (GMCRs) for after vaccination (day 36) versus prevaccination (day 1), proportions of participants with at least two-fold or four-fold rises in antibody titres from baseline at each postvaccination timepoint, and proportions of responders. Among participants who had neutralising antibody titres below the lower limit of quantification (LLOQ) at baseline, responders were defined as those with at least a two-fold increase in titres after vaccination relative to day 1. In participants with baseline titres higher than the LLOQ, responders were those with at least a four-fold increase in titres after vaccination relative to day 1. Prevaccination titres below the assay's LLOQ (1:40) were assigned a value of half the LLOQ.
For cell-mediated immune responses (an exploratory objective), fold rises in individual cytokines at day 36 from day 1 and at day 22 from day 1 were calculated by dividing the day 22 or day 36 measurement by the day 1 measurement; the ratios of fold rises for cytokine pairs (eg, IFNγ to IL-4) and their 95% CIs were computed. Other exploratory immunogenicity objectives, to be presented elsewhere, included the assessment of the ratio of neutralising to binding antibodies and the evaluation of mucosal antibody responses. We also assessed the neutralising antibody responses on day 36 to the beta variant as an exploratory objective.
In a post-hoc analysis, neutralising antibody and binding antibody responses against the D614G variant were measured in a panel of human convalescent-serum samples (Sanguine Biobank, Waltham, MA, USA; iSpecimen, Lexington, MA, USA; PPD, Wilmington, NC, USA; 79 samples) using the same assays that were used on the participant serum samples, in the same laboratory, and within a contemporaneous timeframe to minimise assay variability over time. Convalescent samples were obtained from donors who had recovered from COVID-19 (with clinical severity ranging from mild to severe) and who were asymptomatic at the time of sample collection, as described previously.4 Ratios of vaccine-induced antibody titres to convalescent serum titres were calculated for each antigen-dose group, by age group.
Statistical analysis
All planned analyses were descriptive. A sample size of 160 evaluable participants who were naive to SARS-CoV-2 per group was estimated to enable a minimum observed GMTR between vaccine groups of 0·73, assuming a true GMTR of 1 and a SD of 0·67 (estimated for the pseudovirus neutralisation assay) with 95% probability. Assuming an attrition rate of 15% and capping the proportion of those testing positive by the SARS-CoV-2 rapid serodiagnostic test at 20% of the study population, a total study size of 720 participants (240 in each group) was planned.
Safety endpoints were assessed in the safety analysis set, which included all randomly assigned participants who received at least one dose of the study vaccine and whose data were analysed according to the vaccine actually received. Immunogenicity was assessed in the per-protocol analysis set, which comprised participants who received both injections, provided blood samples at day 1 and day 36, did not have prespecified protocol deviations, and did not receive an authorised COVID-19 vaccine before day 36; data were analysed according to the vaccine group to which participants were randomly assigned. The full analysis set included all participants who received at least one study injection. Cell-mediated immunity was analysed in a randomly selected subset of the per-protocol analysis set. To calculate the proportions of participants with a specific endpoint, the number of participants from the analysis set with data available for that endpoint was used as the denominator.
Predefined subgroup analyses for the main safety parameters were done by age group (18–59 years and ≥60 years), baseline SARS-CoV-2 naive status (naive at day 1 and non-naive at day 1), and high-risk medical conditions (yes or no). Immunogenicity subgroup analyses were done by age group and high-risk medical conditions.
95% CIs for the GMTs, GMCs, GMTRs, and GMCRs were calculated using normal approximation of log-transformed titres. 95% CIs for the proportions of participants with at least two-fold or four-fold increases or responders were calculated with the Clopper-Pearson method.10 95% CIs for the differences in proportions of participants with at least two-fold or four-fold increases and responders were calculated using the Newcombe-Wilson score method without continuity correction.10 Statistical analyses were done using SAS version 9.4 or later.
Role of the funding source
The funders of the study were involved in study design, data collection, data analysis, data interpretation, writing of the report, and the decision to submit the manuscript for publication.
Results
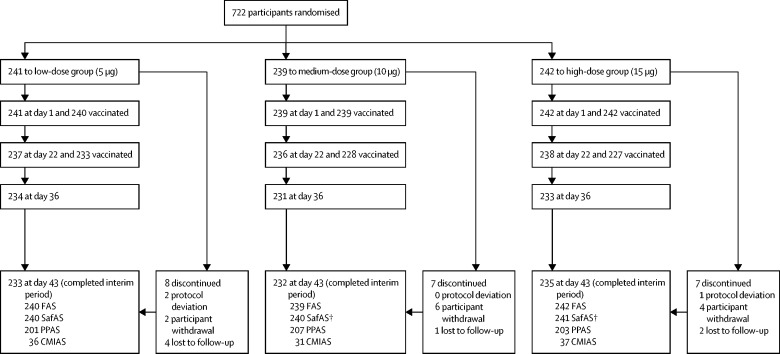
Of 722 participants enrolled and randomly assigned to one of the three study groups between Feb 24, 2021, and March 8, 2021, 721 received at least one injection (low-dose group 240, medium-dose group 239, and high-dose group 242). A total of 22 randomly assigned participants discontinued the study by day 43, none because of an adverse event (figure 1 ). Participants in the safety analysis set were aged 18–95 years (360 aged 18–59 years, 361 aged ≥60 years); baseline demographic characteristics were balanced across treatment groups (table 1 ) and age strata (appendix pp 7–8). Overall, 437 (61%) of 721 participants had at least one high-risk medical condition (full analysis set; appendix p 9).
Figure 1.
Trial profile up to study day 43
CMIAS=cell-mediated immunity analysis set. FAS=full analysis set. SafAS=safety analysis set. PPAS=per-protocol analysis set. No participants discontinued because of an adverse event. † One participant randomly assigned to the high-dose group received medium antigen-dose vaccine formulation on day 0 and received a high-dose formulation as planned on day 22.
Table 1.
Participant demographic characteristics (safety analysis set)
| Low dose (5 μg), n=240 | Medium dose (10 μg), n=240* | High dose (15 μg), n=241* | ||
|---|---|---|---|---|
| Sex | ||||
| Male | 117 (49%) | 126 (53%) | 119 (49%) | |
| Female | 123 (51%) | 114 (48%) | 122 (51%) | |
| Age, years | ||||
| Mean (SD) | 53·8 (15·3) | 53·5 (14·8) | 53·1 (15·9) | |
| Range | 20·0–92·0 | 18·0–88·0 | 19·0–95·0 | |
| Mean (SD) BMI, kg/m2 | 28·4 (5·6) | 28·8 (5·9) | 28·7 (6·0) | |
| Country | ||||
| USA | 192 (80%) | 193 (80%) | 196 (81%) | |
| Honduras | 48 (20%) | 47 (20%) | 45 (19%) | |
| Race | ||||
| White | 156 (65%) | 150 (63%) | 155 (64%) | |
| American Indian or Alaska Native | 22 (9%) | 24 (10%) | 20 (8%) | |
| Black or African American | 13 (5%) | 23 (10%) | 20 (8%) | |
| Asian | 13 (5%) | 10 (4%) | 10 (4%) | |
| Native Hawaiian or other Pacific Islander | 2 (<1%) | 1 (<1%) | 2 (<1%) | |
| Multiple | 5 (2%) | 2 (<1%) | 4 (2%) | |
| Not reported or unknown | 29 (12%) | 30 (13%) | 30 (12%) | |
| Ethnicity | ||||
| Hispanic or Latino | 68 (28%) | 68 (28%) | 67 (28%) | |
| Not Hispanic or Latino | 170 (71%) | 172 (72%) | 173 (72%) | |
| Not reported or unknown | 2 (<1%) | 0 | 1 (<1%) | |
| Baseline SARS-CoV-2 rapid serodiagnostic test | ||||
| Negative | 219 (91%) | 218 (91%) | 219 (91%) | |
| Positive | 21 (9%) | 22 (9%) | 22 (9%) | |
Data are n (%), range, or mean (SD).
One participant randomly assigned to the high-dose group received the medium dose on day 0 and is included in the medium-dose group for the summary of baseline characteristics in the safety analysis set.
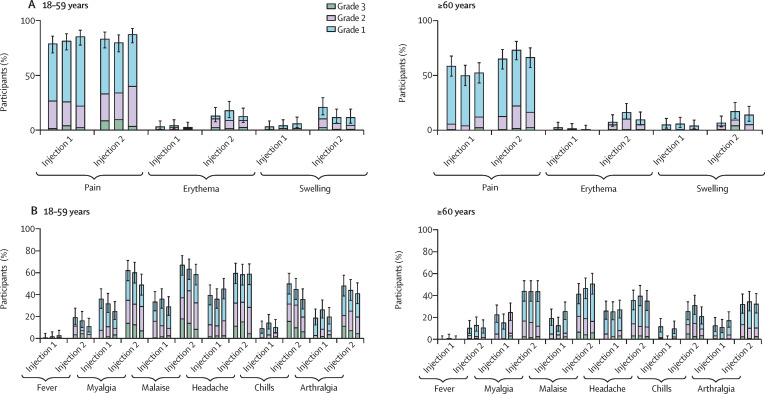
The proportion of participants reporting at least one solicited adverse reaction (injection site or systemic) in the first 7 days after any vaccination was similar between treatment groups, for any intensity (217 [91%] of 238 in the low-dose group, 213 [90%] of 237 in the medium-dose group, and 218 [91%] of 239 in the high-dose group) and for grade 3 intensity (52 [22%] of 238 in the low-dose group, 49 [21%] of 237 in the medium-dose group, and 45 [19%] of 239 in the high-dose group; appendix pp 10–12). The most frequently reported solicited injection-site reaction was injection-site pain (figure 2A ), and the most frequently reported solicited systemic reactions were malaise, headache, and myalgia (figure 2B). Grade 3 solicited reactions were transient, with most occurring on the day of, or the day after, vaccination (appendix pp 28–31) and resolving within 2 days without requiring medical attention.
Figure 2.
Solicited injection-site (A) and systemic (B) adverse reactions up to 7 days after each injection, by age group (safety analysis set)
Error bars show 95% CIs. Each group of three bars represent low-dose (5 7mu;g), medium-dose (10 7mu;g), and high-dose (15 7mu;g) groups (from left to right).
Overall, four immediate unsolicited adverse events were reported in four participants (appendix pp 10–12): two assessed by the investigator to be vaccine related (grade 1 lymphadenopathy in the low-dose group and grade 1 paraesthesia in the medium-dose group, on the same side as the injection site) and two to be unrelated (grade 1 presyncope in the low-dose group and grade 3 hypertension in the high-dose group). The case of lymphadenopathy resolved with medication within 5 days, whereas the other adverse events resolved spontaneously within 1 day.
The proportion of participants reporting at least one unsolicited adverse event (or adverse reaction) up to 21 days after any vaccination was similar across antigen dose groups (appendix pp 10–12). Grade 3 unsolicited adverse events were reported most frequently in the high-dose group (19 [8%] of 241 vs five [2%] of 240 in the low-dose group and six [3%] of 240 in the medium-dose group), as were grade 3 unsolicited adverse reactions (one [<1%] of 240 in the low-dose group, three [1%] of 240 in the medium-dose group, and five [2%] of 241 in the high-dose group). Unsolicited adverse events and adverse reactions tended to be reported more frequently among younger adults than older adults in the low-dose and medium-dose groups, but not in the high-dose group (appendix pp 10–12). The majority of unsolicited adverse reactions were compatible with reactogenicity symptoms (appendix p 13), were of grade 1 or 2 intensity, occurred within the first 4 days after injection, and generally resolved within 7 days.
Six participants in the high-dose group and one in the low-dose group reported unsolicited adverse events with the MedDRA preferred terms of elevated blood pressure, elevated systolic blood pressure, essential hypertension, or hypertension. These unsolicited adverse events occurred shortly after vaccination, self-resolved within 1–2 days, and occurred without any other associated symptoms in all but one case; one participant with grade 3 hypertension had macular rash and headache (all assessed to be related to the study vaccine) and anxiety (assessed as unrelated). Medically attended adverse events were reported in 62 (9%) of 721 participants, with no clear difference between treatment groups (appendix pp 10–12). Of these, seven grade 3 adverse events (reported by five participants) were assessed by the investigator to be related to the vaccine (two in the low-dose group, one in the medium-dose group, and four in the high-dose group) and three (not related) were assessed to be serious (two in the medium-dose group and one in the high-dose group). Grade 3 medically attended adverse events tended to be more frequent in the high-dose group (eight [3%] of 241) than in the low-dose group (four [2%] of 240) and medium-dose group (two [<1%] of 240); these grade 3 events included two events of grade 3 hypertension assessed as related to the study vaccine. No adverse events led to study discontinuation, and no adverse events of special interest were reported. Four SAEs were reported (two in each of the medium-dose and high-dose groups), none of which were considered by the investigator or the sponsor to be related to the study vaccine. Solicited reactions and unsolicited adverse events and reactions tended to be reported less frequently in participants with at least one high-risk medical condition compared with those without any high-risk medical condition (appendix pp 17–19). The safety and reactogenicity profiles were similar between participants who were SARS-CoV-2 naive at day 1 and those who were non-naive at day 1 (appendix pp 14–16).
Among 611 participants in the per-protocol analysis set, 598 (98%) had sufficient information to determine SARS-CoV-2 naive status at day 1 and day 22: 521 (85%) were naive and 77 (13%) non-naive. The numbers of naive and non-naive participants were balanced across treatment groups: 168 (84%) of 201 participants in the low-dose group, 177 (86%) of 207 in the medium-dose group, and 176 (87%) of 203 in the high-dose group were naive, and 28 (14%) in the low-dose group, 26 (13%) in the medium-dose group, and 23 (11%) in the high-dose group were non-naive.
Among participants in the per-protocol analysis set who were SARS-CoV-2 naive, neutralising antibody GMTs to the D614G variant 14 days after the second injection (day 36) were 2189 (95% CI 1744–2746) in the low-dose group, 2269 (1792–2873) in the medium-dose group, and 2895 (2294–3654) in the high-dose group. GMTRs comparing day 36 with day 1 were 107 (95% CI 85–135) in the low-dose group, 110 (87–140) in the medium-dose group, and 141 (111–179) in the high-dose group. 158 (98%) of 162 participants were responders (two-fold or greater increase in neutralising antibody titre from baseline) in the low-dose group versus 166 (99%) of 168 in the medium-dose group and 163 (98%) of 166 in the high-dose group (table 2 ).
Table 2.
Neutralising antibody responses to D614G by age group 14 days after the second injection (day 36) in SARS-CoV-2-naive participants (per-protocol analysis set)
| Low dose (5 μg; n=168) | Medium dose (10 μg; n=177) | High dose (15 μg; n=176) | Convalescent sera (n=79)* | |
|---|---|---|---|---|
| All ages | ||||
| ≥2-fold rise (responders) | 97·5% (93·8–99·3; 158/162) | 98·8% (95·8–99·9; 166/168) | 98·2% (94·8–99·6; 163/166) | .. |
| ≥4-fold rise | 96·9% (92·9–99·0; 157/162) | 97·0% (93·2–99·0; 163/168) | 97·6% (93·9–99·3; 162/166) | .. |
| GMT | 2189 (1744–2746; 165) | 2269 (1792–2873; 173) | 2895 (2294–3654; 172) | 2140 (1543–2967) |
| GMTR | 107 (85·1–135; 162) | 110 (86·6–140; 168) | 141 (111–179; 166) | .. |
| 18–59 years | ||||
| ≥2-fold rise (responders) | 100% (95·5–100; 80/80) | 97·4% (91·0–99·7; 76/78) | 100% (95·5–100; 80/80) | .. |
| ≥4-fold rise | 100% (95·5–100; 80/80) | 97·4% (91·0–99·7; 76/78) | 100% (95·5–100; 80/80) | .. |
| GMT | 2954 (2272–3840; 82) | 3951 (2851–5474; 81) | 5142 (3800–6958; 81) | .. |
| GMTR | 146 (112–190; 80) | 192 (137–269; 78) | 261 (192–354; 80) | .. |
| ≥60 years | ||||
| ≥2-fold rise (responders) | 95·1% (88·0–98·7; 78/82) | 100% (96·0–100; 90/90) | 96·5% (90·1–99·3; 83/86) | .. |
| ≥4-fold rise | 93·9% (86·3–98·0; 77/82) | 96·7% (90·6–99·3; 87/90) | 95·3% (88·5–98·7; 82/86) | .. |
| GMT | 1628 (1132–2341; 83) | 1393 (1021–1899; 92) | 1736 (1264–2385; 91) | .. |
| GMTR | 79·2 (55·0–114; 82) | 68·1 (49·7–93·2; 90) | 79·9 (57·9–110; 86) | .. |
Data are % (95% CI; number of responders/number of participants with data available), GMT (95% CI; number of participants with data available), or GMTR (95% CI; number of participants with data available). GMT=geometric mean titre. GMTR=geometric mean titre ratios (day 36 vs day 1). n=total number of participants who were SARS-CoV-2 naive on days 1 and 22.
Neutralising antibodies measured in a panel of sera obtained from donors who had recovered from COVID-19 and were asymptomatic at the time of sample collection.
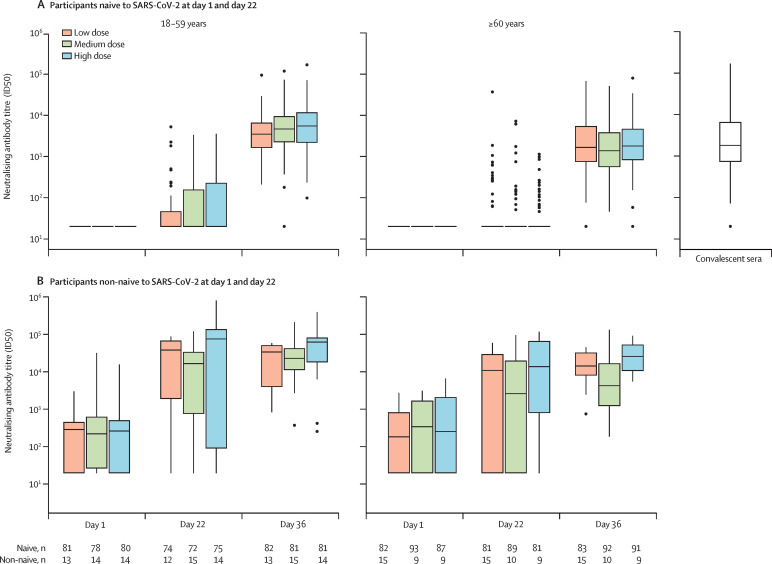
Neutralising antibody titres after the second injection (day 36) tended to increase with antigen dose in the younger age group but not in older adults; titres were higher for younger adults than for older adults within each dose group (figure 3A ), whereas the proportions of participants with at least two-fold or four-fold rises in neutralising antibody titres were similar between the age groups (table 2). In a post-hoc analysis, the magnitude of neutralising antibody titres at day 36 were similar to titres observed in the convalescent sera panel (2140, 95% CI 1543–2967; table 2), with ratios of vaccine-induced neutralising-antibody titres to titres in the convalescent panel of 1·38, 1·85, and 2·40 among younger adults and 0·76, 0·65, and 0·81 among older adults for the low-dose, medium-dose, and high-dose groups, respectively. On day 22 after the first injection, neutralising antibody titres showed minimal increases from baseline, regardless of antigen dose group, for both age strata (figure 3A; appendix p 20).
Figure 3.
Neutralising antibody response to D614G, after each injection, by SARS-CoV-2 naive status (per-protocol analysis set)
Boxes indicate median and quartile ranges. Outliers are plotted as individuals points. (A) Where the 75th percentile of neutralisation ID50 titres could not be distinguished from the other two percentile values, boxes with medians and IQRs could not be provided. Number of participants available for each endpoint are shown in the table. 79 convalescent sera samples were available. The lower limit of quantification of the pseudovirus neutralising-antibody assay was 1/40, with an upper limit of 1/191 429.
Among participants who were SARS-CoV-2 naive with at least one high-risk medical condition, neutralising antibody titres to the D614G variant were similar across antigen-dose groups in both age strata. Among participants who were naive without high-risk medical conditions, higher neutralising-antibody titres with increasing antigen dose was observed in younger adults. After two doses, neutralising antibody titres among those without high-risk conditions were higher than those with at least one high-risk medical condition in the medium-dose and high-dose groups, particularly in the younger age stratum; this finding was not observed in the low-dose group (appendix p 21).
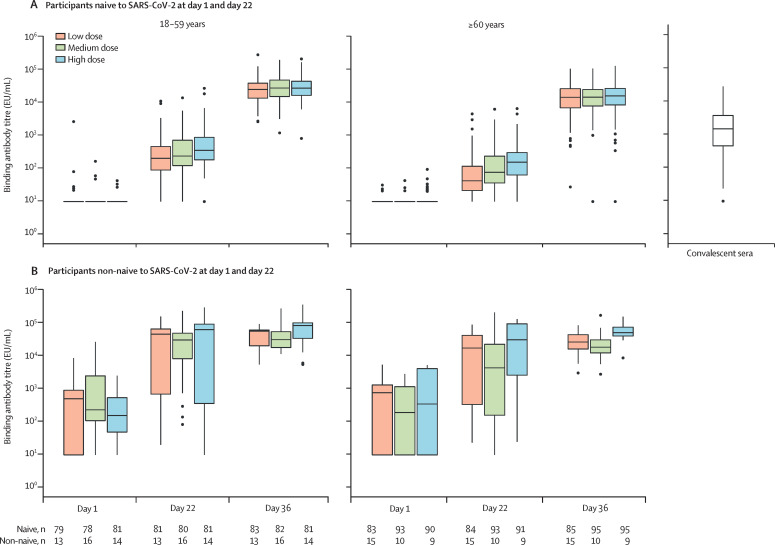
Binding antibody responses 14 days after the second injection (day 36) among naive participants in the per-protocol analysis set were high across antigen-dose groups, with minimal increases observed after the first injection (day 21) and higher binding-antibody concentrations in the younger age group than in the older age group for each antigen-dose group (appendix pp 22–23; figure 4A ).
Figure 4.
Binding antibody response to D614G, following each injection, by SARS-CoV-2 naive status (per-protocol analysis set)
Boxes indicate median and quartile ranges. Outliers are plotted as individuals points. Number of participants available for each endpoint are shown in the table. 78 convalescent sera samples were available. The lower limit of quantification of the SARS-CoV-2 anti-spike protein IgG ELISA was 18·9 EU/mL, with an upper limit of 115 008·0 EU/mL.
Among participants in the per-protocol analysis set who were non-naive at day 1 or day 22, or both, neutralising antibody titres (figure 3B; appendix pp 24–25) and binding antibody concentrations (figure 4B) to the D614G variant increased more than ten times in both age strata 21 days after a single injection (day 22) in all antigen dose groups, such that in each antigen dose group the day 22 titres in participants who were non-naive were higher than those reached among participants who were naive after two doses (day 36). Higher titres and greater increases were observed in the younger age stratum than in the older age stratum. GMTs increased further after the second injection, albeit to a lesser extent, with nearly all participants in each group achieving at least a four-fold rise in neutralising antibody titres and binding antibody concentrations by day 36 (appendix pp 22–25).
The neutralising antibody response to the beta variant was assessed at day 36 only (appendix pp 26, 32). In naive participants in the per-protocol analysis set, GMTs were similar between the low-dose and medium-dose groups, and slightly higher in the high-dose group, with titres approximately ten times lower than for the D614G variant. The pattern of neutralising antibody responses to the beta variant with age was similar to that observed with responses to the D614G variant, with higher titres in younger adults than older adults. In participants who were non-naive, the beta-variant GMTs were also similar between the low-dose and medium-dose groups, with higher titres for the high-dose group (appendix pp 26 and 32).
Of 120 participants randomly assigned for assessment of cell-mediated immunity, data were available for a subset of 104: 36 participants in the low-dose group, 31 in the medium-dose group, and 37 in the high-dose group. An increase in Th1 and Th2 cytokines was observed after vaccination, with a higher increase in cytokines after the second injection than after the first (appendix p 27). Increases in IFNγ, IL-2, and TNFα cytokines from before vaccination to day 22 and day 36 were greater than the increases for IL-4, IL-5, and IL-13, with ratios of Th1:Th2 cytokines higher than 1, suggesting no Th2-cell bias in the cell-mediated responses (appendix pp 33–34).
Discussion
In this study, two injections of the AS03-adjuvanted SARS-CoV-2 recombinant-protein vaccine, CoV2 preS dTM-AS03, showed an acceptable safety and reactogenicity profile, and favourable neutralising antibody and cellular immune responses in adults who were SARS-CoV-2 naive and non-naive, for all three antigen dose groups, and in both younger (18–59 years) and older (≥60 years) age strata.
No safety concerns were identified during the interim study period. In the current study, local and systemic solicited reactions were reported more frequently after the second injection and in the younger age strata, consistent with our previous observations4 and with other COVID-19 vaccines.11, 12, 13, 14 Solicited adverse reactions were reported less frequently and were milder with the optimised formulations in the current study than with the formulations tested in the previous phase 1–2 trial.4 We observed a similar reactogenicity profile between individuals who were naive and non-naive, by contrast with reports from other vaccines of higher rates of solicited reactions in seropositive vaccinees.15, 16 AS03-adjuvanted vaccines have consistently shown increased reactogenicity compared with the corresponding unadjuvanted vaccines, for pandemic influenza vaccines17 and for CoV2 preS dTM formulations investigated in our previous phase 1–2 study.4 Of note, the proportions of participants with local and systemic adverse reactions after two vaccine doses were higher in our study than previously observed with AS03-adjuvanted pandemic influenza vaccines18, 19 and in phase 1 trials of the AS03-adjuvanted SARS-CoV-2 virus-like particle vaccine14 and the AS03-adjuvanted recombinant full-length S protein vaccine produced in CHO cells;13 however, the proportions were similar to those observed in the clinical study of the SARS-CoV-2 mRNA-1273 vaccine after two vaccine doses.20 Although our study did not include a placebo group, which might affect the reporting of reactogenicity, these observations taken together suggest that the combination of the adjuvant and antigen contribute to the reactogenicity profile of candidate SARS-CoV-2 vaccines. Transient, self-resolving events of elevated blood pressure not associated with symptoms (except in one participant) were observed shortly after vaccination, which could be consistent with a procedure-related noradrenergic discharge around the time of vaccination.21, 22
Almost all (≥97%) participants who were SARS-CoV-2 naive attained a four-fold rise in neutralising antibody titres to the D614G variant at day 36, regardless of age strata, presence of high-risk medical condition, or antigen dose. The magnitude of the neutralising antibody response observed at day 36 in the naive study population was similar to that observed for a panel of human convalescent sera. Early phase studies of other candidate SARS-CoV-2 vaccines have also shown similar results for vaccine-elicited antibody titres and those measured in convalescent plasma samples,23, 24, 25, 26 which supported their further clinical development to efficacy trials; however, direct comparisons with other SARS-CoV-2 vaccines are not possible at this time because different laboratories and different assays were used. Among adults who were non-naive in our study, a single injection increased D614G neutralising-antibody titres to concentrations higher than those observed after two injections in adults who were naive and exceeded those measured in the convalescent sera. Our findings are in line with the robust antibody responses previously observed after a single dose of the BNT162b2 or mRNA-1273 SARS-CoV-2 vaccines in patients who were SARS-CoV-2 seropositive.16, 27, 28, 29 It is interesting to note the variability in responses after the first dose in participants who were non-naive, potentially because of the variability in previous infection and priming, as well as in the duration of the interval between infection and vaccination.
Information regarding correlates of protection is scarce.30 However, recent work has modelled the correlation between the ratio of neutralising antibody responses in vaccinees to convalescent sera and the observed vaccine efficacy to account for differences across assays and convalescent sera.31, 32 In these models, ratios of 1 correlate with vaccine efficacy of 80–90% and ratios of 0·8 correlate with vaccine efficacy of 70–80%. These models were based on neutralising antibody responses and efficacy against homologous variants, or variants with small drifts. In the current study, the ratio of neutralising antibody titres to convalescent sera ranged from 1·38 to 2·40 across groups among the younger adults who were naive, and between 0·65 and 0·81 among the older adults who were naive. The lower responses to the beta variant seen in our study, consistent with data from other authorised or investigational COVID vaccines,33, 34, 35 suggest neutralising antibody titres and predicted vaccine efficacy against heterologous variants are likely to be lower.32 These comparisons with convalescent sera should be interpreted with caution because they are exploratory and we have scarce information on the donors of the convalescent sera used in this study.
On the basis of the interim data described here, the CoV2 preS dTM-AS03 candidate vaccine has progressed to phase 3 efficacy evaluation (NCT04904549). As the reactogenicity and safety profiles were similar across antigen dose groups, the choice of antigen dose to progress to phase 3 efficacy evaluation was largely dependent on the observed immunogenicity profile in adults who were naive. The selection of a 10 7mu;g S antigen dose for a monovalent vaccine, over the 5 7mu;g dose, might mitigate the potential effect of variant circulation because it provides higher cross-reactive antibody titres against variant strains in individuals who are naive, albeit we did not observe a clear dose–response relationship to the beta variant. On the basis of the above-mentioned models of predicted vaccine efficacy curves, we would expect any potential difference in vaccine protection between antigen dose groups to be limited. Furthermore, in the context of a pandemic, a lower antigen dose would translate into a substantial increase in vaccine supply. In the phase 3 study, a bivalent AS03-adjuvanted vaccine containing 5 7mu;g D614G antigen and 5 7mu;g beta antigen is being evaluated. Because the 5 7mu;g dose in the naive population in this study provided homologous neutralising-antibody responses similar to convalescent sera, it is expected that a similar homologous response would be elicited by the beta component of a bivalent vaccine.
Fractionation of doses has been suggested as an important strategy for meeting global vaccine demand,36 particularly for booster vaccines. The robust neutralising antibody responses observed after a single injection of the 5 7mu;g antigen-dose formulation in participants who were SARS-CoV-2 non-naive suggest that a single dose of 5 7mu;g CoV2 preS dTM antigen with AS03 adjuvant might be sufficient for boosting previously primed individuals. This phase 2 study has been amended to include cohorts of previously vaccinated individuals to evaluate a single 5 7mu;g antigen dose as a booster vaccine.
The number of participants in this study limited the assessment of rare SAEs and adverse events of special interest, although continued follow-up and the large sample size recruited for the subsequent phase 3 study will provide a robust dataset for further safety evaluation. Although we report neutralising antibody responses to the beta variant, a major variant of concern at the time we designed the study, we acknowledge that we have not evaluated neutralising antibody responses to either the delta variant or the omicron variant, which have since become the dominant circulating variants of concern. Other limitations include that information on the durability of the immune response is not available from this interim analysis, and that antigen doses lower than 5 7mu;g, which could be of interest for boosting primed individuals, were not evaluated here.
In summary, two doses of the CoV2 preS dTM-AS03 vaccine candidate showed an acceptable safety profile and robust immunogenicity in adults who were naive to SARS-CoV-2, including in individuals aged 60 years and older and those with high-risk medical conditions. On the basis of these results, two formulations of the CoV2 preS dTM-AS03 vaccine candidate, a monovalent D614G and a bivalent D614G and a beta variant vaccine, are undergoing efficacy evaluation in phase 3 trials. Furthermore, the high neutralising titres and acceptable safety after a single vaccine dose observed in participants with evidence of previous SARS-CoV-2 infection indicate the possibility of developing a formulation with a lower antigen dose, and a single-dose vaccination strategy, for use as a booster for adults who have been previously primed.
Data sharing
Qualified researchers can request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case-report forms, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Declaration of interests
SSr, MIB, A-LC, AC, RMC, BF, HJ, YM, ER, NR, DvB, GdB, RC, M-HG, DL, RM, SM, CM, CAD, and SSa are Sanofi Pasteur employees. SSr, MIB, HJ, CM, and SSa own stock or stock options; A-LC, NR, and M-HG hold shares or restricted shares; and RMC reports owning shareholder and vesting options in Sanofi Pasteur. SSr, RMC, GdB, CAD, and SSa are inventors on a pending patent application filed by Sanofi Pasteur and GlaxoSmithKline (GSK) for the development of the CoV-2 dTM vaccine. LS, MK, MAC, and FT-D-S are employed by, and hold restricted shares in, the GSK group of companies. AJ was contracted by Sanofi Pasteur. DD received funding from Sanofi Pasteur for his institution, George Washington University, to serve as a clinical site for the vaccine trial. NG reports receiving funding and study materials from Sanofi and funding from the National Institute of Allergy and Infectious Diseases (NIAID) through the COVID-19 Prevention Network (CoVPN), which provides research grants to her institution, the Fred Hutchinson Cancer Research Center. MCK received NIAID funding support for the current study, paid to the clinical trials site for the CoVPN and HIV vaccine trials network; Sanofi Pasteur contracted the site to do this clinical trial. DMRM received a research grant from Sanofi Pasteur for this study. OO received a Gilead Sciences grant paid to Yale, and consulting fees from Gilead Sciences. OO also reports that Gilead, Integrity CE, and Medscape paid for lectures, presentations, manuscript writing, or educational events and that Gilead and ViiV Healthcare paid for their participation on a data safety monitoring or advisory board. VNR received funding from Sanofi for the CoVPN and Vaccine Treatment and Evaluation Units for COVID-19 vaccine clinical trials, and payments from Pfizer to her institution for the conduct of COVID-19 vaccine clinical trials. RS received clinical trial investigator site contracts with AES Synexus. NGR received a grant from Sanofi Pasteur to do this study and received grants from Sanofi, Merck, Pfizer, Quidel, and Lilly in the past 36 months. NGR received a payment from the Infectious Disease Association of California and participated on advisory or safety monitoring boards for Micron, ICON, and The Emmes Company; NGR also reports having a leadership or fiduciary role (paid or unpaid) for the Infectious Diseases Society of America, the Vaccine Treatment and Evaluation Unit and Antibacterial Resistance Leadership Group. LDS received a research grant from Sanofi Pasteur and SRW and JW were contracted by Sanofi Pasteur. AB, BJE, NLM, SSM, MKJ, SR, JS, TT, and JT declare no competing interests.
Acknowledgments
Acknowledgments
The authors thank all participants, investigators, and study site personnel who took part in this study. The authors acknowledge Juliette Gray of inScience Communications, Springer Healthcare, London, UK, for providing editorial assistance with the preparation of this manuscript, funded by Sanofi Pasteur. The authors also thank Isabel Grégoire for providing editorial assistance and manuscript coordination on behalf of Sanofi Pasteur. This study was funded by Sanofi Pasteur and by federal funds from the Biomedical Advanced Research and Development Authority, part of the office of the Assistant Secretary for Preparedness and Response at the US Department of Health and Human Services in collaboration with the US Department of Defense Joint Program Executive Office for Chemical, Biological, Radiological, and Nuclear Defense under contract number W15QKN-16-9-1002. The views presented here are those of the authors and do not purport to represent those of the Department of the Army. This work was done in collaboration with GlaxoSmithKline, who provided access to, and use of, the AS03 adjuvant system.
Contributors
SSr, MIB, A-LC, RMC, BF, NAG, YM, NLM, NGR, LS, M-HG, MK-J, MK, TT, JT, CAD, and SSa contributed to the concept or design of the study and data analysis and interpretation. HJ contributed to the conception and design of the study. SRW contributed to the concept and design of the study, and the data analysis, interpretation, and acquisition. GdB and JS were involved in data acquisition, analysis, and interpretation. AJ, AB, DD, BJE, MCK, DMRM, SSM, OO, VNR, RS, ER, NR, LDS, JW, DvB, RC, DL, SM, CM, SR, and AS contributed to data acquisition. AC, NR, MAC, and FT-D-S were involved in the analysis and interpretation of the data. RM was involved in the conception and design of the study and data acquisition. All authors were involved in drafting or critically revising the manuscript, and all authors approved the final version and are accountable for the accuracy and integrity of the manuscript. All authors had full access to all the data and accept responsibility to submit for publication. At least two authors (AC and BF) have accessed and verified the data.
Supplementary Material
References
- 1.World Bank . World Bank; Washington, DC: 2021. Global economic prospects, June 2021. [Google Scholar]
- 2.WHO The impact of COVID-19 on global health goals. https://www.who.int/news-room/spotlight/the-impact-of-covid-19-on-global-health-goals
- 3.Regulatory Affairs Professionals Society COVID-19 vaccine tracker. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker
- 4.Goepfert PA, Fu B, Chabanon AL, et al. Safety and immunogenicity of SARS-CoV-2 recombinant protein vaccine formulations in healthy adults: interim results of a randomised, placebo-controlled, phase 1–2, dose-ranging study. Lancet Infect Dis. 2021;21:1257–1270. doi: 10.1016/S1473-3099(21)00147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arunachalam PS, Walls AC, Golden N, et al. Adjuvanting a subunit COVID-19 vaccine to induce protective immunity. Nature. 2021;594:253–258. doi: 10.1038/s41586-021-03530-2. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention Underlying medical conditions associated with high risk for severe COVID-19: information for healthcare providers. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html [PubMed]
- 7.Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an adjuvant system containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vacc. 2012;11:349–366. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 8.Petropoulos CJ, Parkin NT, Limoli KL, et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicr Agent Chemother. 2000;44:920–928. doi: 10.1128/aac.44.4.920-928.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavares Da Silva F, De Keyser F, Lambert P-H, Robinson WH, Westhovens R, Sindic C. Optimal approaches to data collection and analysis of potential immune mediated disorders in clinical trials of new vaccines. Vaccine. 2013;31:1870–1876. doi: 10.1016/j.vaccine.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 10.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. New Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 13.Richmond P, Hatchuel L, Dong M, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet. 2021;397:682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward BJ, Gobeil P, Séguin A, et al. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat Med. 2021;27:1071–1078. doi: 10.1038/s41591-021-01370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krammer F, Srivastava K, Simon V. Robust spike antibody responses and increased reactogenicity in seropositive individuals after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohet C, van der Most R, Bauchau V, et al. Safety of AS03-adjuvanted influenza vaccines: a review of the evidence. Vaccine. 2019;37:3006–3021. doi: 10.1016/j.vaccine.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Jackson LA, Campbell JD, Frey SE, et al. Effect of varying doses of a monovalent H7N9 influenza vaccine with and without AS03 and MF59 adjuvants on immune response: a randomized clinical trial. JAMA. 2015;314:237–246. doi: 10.1001/jama.2015.7916. [DOI] [PubMed] [Google Scholar]
- 19.Levie K, Leroux-Roels I, Hoppenbrouwers K, et al. An adjuvanted, low-dose, pandemic influenza A (H5N1) vaccine candidate is safe, immunogenic, and induces cross-reactive immune responses in healthy adults. J Infect Dis. 2008;198:642–649. doi: 10.1086/590913. [DOI] [PubMed] [Google Scholar]
- 20.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. New Engl J Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Immunization stress-related responses: a manual. 2019. https://www.who.int/publications/i/item/978-92-4-151594-8
- 22.Meylan S, Livio F, Foerster M, Genoud PJ, Marguet F, Wuerzner G. Stage III hypertension in patients after mRNA-based SARS-CoV-2 vaccination. Hypertension. 2021;77:e56–e57. doi: 10.1161/HYPERTENSIONAHA.121.17316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Formica N, Mallory R, Albert G, et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2: preliminary report. New Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med. 2021;384:1824–1835. doi: 10.1056/NEJMoa2034201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manisty C, Otter AD, Treibel TA, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prendecki M, Clarke C, Brown J, et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadat S, Tehrani ZR, Logue J, et al. Single dose vaccination in healthcare workers previously infected with SARS-CoV-2. JAMA. 2021;325:1467–1469. doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. 2021 doi: 10.1101/2021.08.09.21261290. published online Aug 15. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khoury DS, Cromer D, Reynaldi A, et al. What level of neutralising antibody protects from COVID-19? Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 33.Liu Y, Liu J, Xia H, et al. Neutralizing activity of BNT162b2-elicited serum. New Engl J Med. 2021;384:1466–1468. doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madhi SA, Baillie V, Cutland CL, et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B.1.351 variant. New Engl J Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann M, Arora P, Groß R, et al. SARS-CoV-2 variants B.1.351 and P.1 escape from neutralizing antibodies. Cell. 2021;184:2384. doi: 10.1016/j.cell.2021.03.036. 93.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cowling BJ, Lim WW, Cobey S. Fractionation of COVID-19 vaccine doses could extend limited supplies and reduce mortality. Nat Med. 2021;27:1321–1323. doi: 10.1038/s41591-021-01440-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers can request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case-report forms, statistical analysis plan, and dataset specifications. Patient-level data will be anonymised and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data-sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.