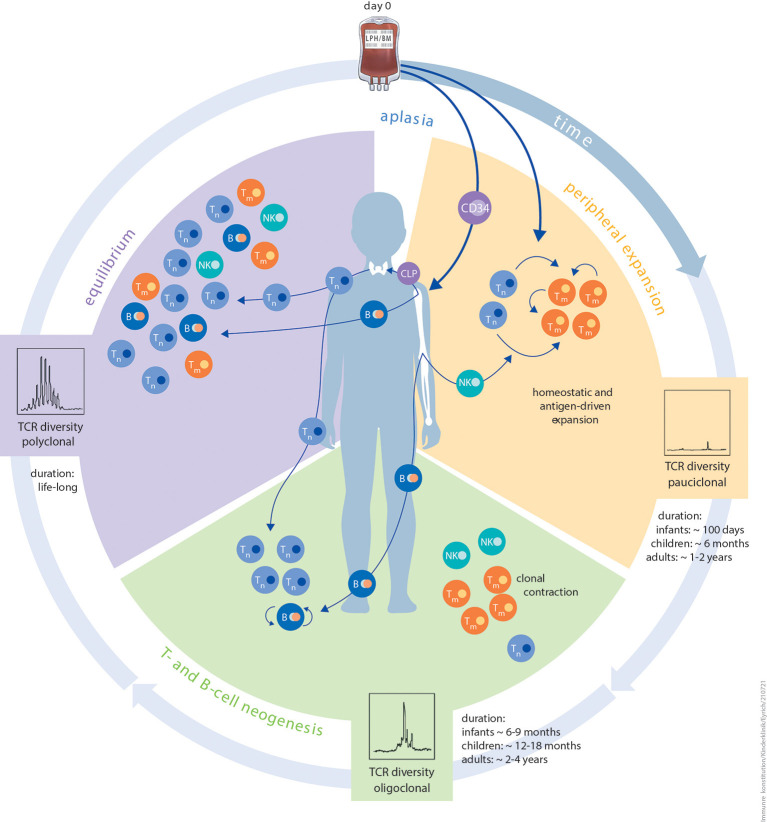
Figure 1.
Schematic illustration of the different phases of immune reconstitution following HCT. The first phase peripheral expansion (orange) of IR after aplasia is dominated by homeostatic or antigen-driven peripheral expansion of graft-derived T cells. The ratio of naïve T cells to memory T cells is dependent on donor age. The quantity of regenerating T-cell numbers depends on graft size (bone marrow vs. PBSC) and in vivo (serotherapy) or in vitro T-cell depletion. Diversity of the TCR repertoire during this phase is usually dominated by expansion of singular clonotypes. The duration of this period is strictly influenced by patient age. The second phase T- and B-cell neogenesis (green) of IR is characterised by the onset of T- and B-cell neogenesis in the thymus and bone marrow. Thymic and bone marrow niches are more resilient against external stressors and more productive in infants and children than in adults. Other contributing factors are thymic tissue status, application of immunosuppression, and aGvHD or cGvHD. The risk of viral reactivation dramatically reduces as T- and B-cell neogenesis advances. The same probably applies to de novo GvHD. In this phase, immunisation with non-live vaccines is feasible. The third and final phase equilibrium (purple) of IR is a balanced and stable immune system, which is, to the best of our knowledge, maintained lifelong. Components of innate as well as adaptive immunity reach a level that is relative to patient age. Diversity of the TCR repertoire is polyclonal at this phase. Live, attenuated vaccines can be applied since positive T-cell and B-cell interactions are granted. Autoantibodies tend to disappear and risk of cGvHD is minimal. B, B cell; CLP, common lymphoid progenitor; NK, natural killer cell; TCR, T-cell receptor; Tm, memory T cell; Tn, naïve T cell.