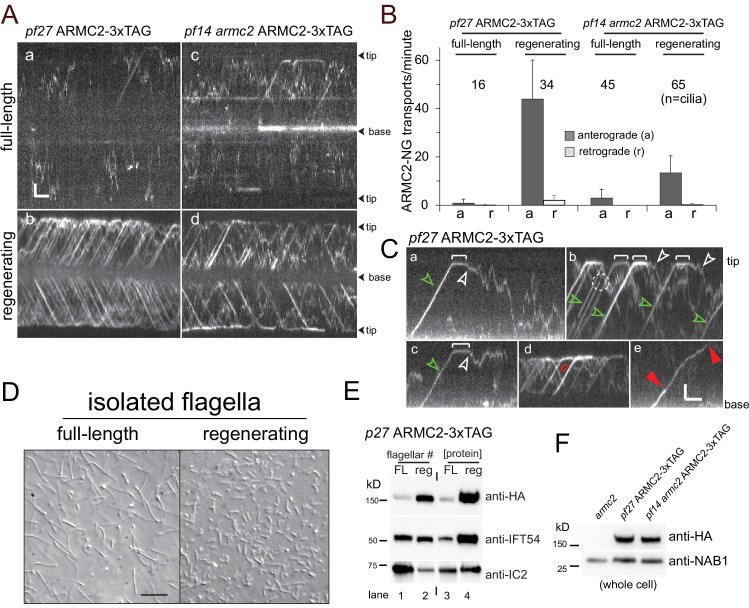
Figure 2. ARMC2-3xTAG is highly enriched in regenerating flagella.
(A) TIRF imaging of ARMC2-3xTAG in the pf27 background (a, b) and the pf14 armc2 double mutant background (c, d) in full-length (a, c) and in regenerating flagella (b, d). Bars = 2 s 2 µm. The flagellar tips and bases are indicated. (B) Bar graph showing the average frequencies (events/min/flagellum) of anterograde and retrograde transport of ARMC2-3xTAG in full-length and regenerating flagella of the pf27 ARMC2-3XTAG and the pf14 armc2 ARMC2-3xTAG strain. The standard deviation and the number of flagella analyzed are indicated. (C) Kymograms of ARMC2-3xTAG in late regenerating pf27 flagella. The white brackets in a–c mark the dwell time of individual ARMC2-3xTAG particles between arrival at the tip by anterograde IFT and the onset of diffusion (white arrowheads). Green arrowheads in a–c, anterograde transport of ARMC2-3xTAG, red open arrow in d, retrograde IFT of ARMC2-3xTAG; red arrowheads in e, stepwise bleaching of ARMC2-3xTAG indicating for the presence of two copies. In c, a single step bleaching event is marked by a dashed circle. Bars = 2 s and 2 µm. (D) DIC images of full-length and regenerating flagella of the pf27 ARMC2-3xTAG strain. Regenerating flagella were harvested ~22 min after deflagellation by a pH shock. Bar = 10 µm. (E) Western blot analysis of the full-length and regenerating flagella shown in C with the antibodies indicated. On the left side, an equal number of flagella were loaded and on the right side, approximately equal loading of protein was attempted. (F) Western blot comparing the presence of ARMC2-3xTAG in the pf27 ARMC2-3xTAG and the pf14 armc2 ARMC2-3xTAG strain. Antibodies to the cell body protein nucleic acid binding protein 1 (NAB1) were used as a loading control.