Figure 7.
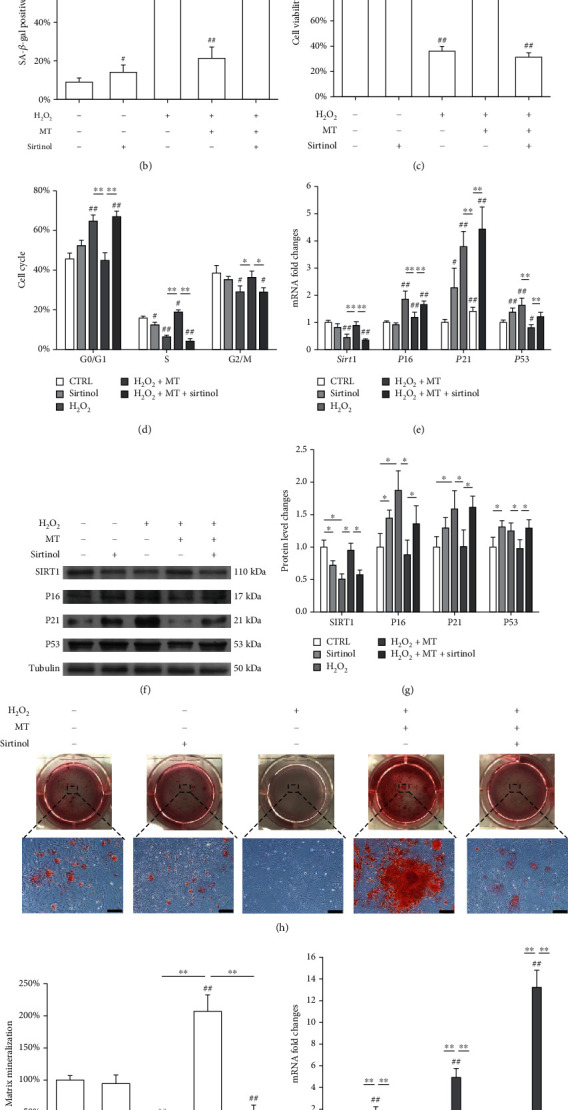
Inhibition of SIRT1 by sirtinol counteracted the antisenescence effects of melatonin on OVX BMMSCs. In vitro cultured OVX BMMSCs were first exposed to H2O2 for 2 h and then treated with 100 μM of melatonin (MT) with or without sirtinol (40 μM) for 72 h. (a, b) Senescent cells were labeled by senescence-associated β-galactosidase (SA-β-gal) staining. Scale bar = 100 μm. (c) The effect of sirtinol treatment on cell viability was determined by CCK-8 assays. (d) The effect of sirtinol treatment on cell cycle distribution was evaluated by flow cytometry. (e) The mRNA expression levels of Sirt1, P16, P21, and P53 were quantified using qRT-PCR. (f, g) The protein levels of SIRT1, P16, P21, and P53 were determined using Western blot assays. (h) Sirtinol-treated BMMSCs were induced toward osteogenic differentiation. Matrix mineralization was determined by Alizarin Red S (ARS) staining. Scale bar = 200 μm. (i) The stained mineral layers were quantified. The values were normalized to those of the sham group. (j) The mRNA levels of osteoblast-specific marker genes, including Runx2, Sp7, and Bglap, were quantified with qRT-PCR in which Gapdh was used for normalization. Values are presented as the mean ± S.E.M of six independent experiments (n = 6) in SA-β-gal staining, eight independent experiments (n = 8) in cell viability assays, three independent experiments (n = 3) in cell cycle assays, four independent experiments (n = 4) in ARS assays, and four independent experiments (n = 4) in qRT-PCR experiments, and three independent experiments (n = 3) in Western blot assays. Statistically significant differences are indicated by ∗p < 0.05 or ∗∗p < 0.01 between the indicated groups; #p < 0.05 or ##p < 0.01 versus the sham group.
