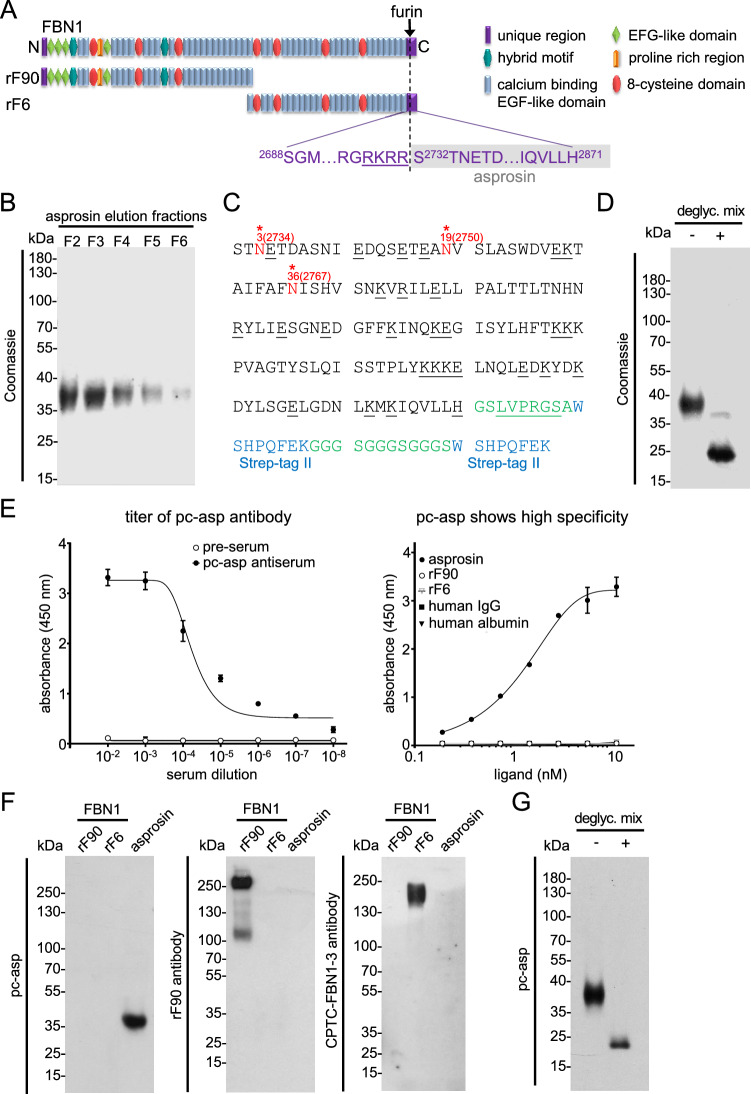
Figure 1.
Generation of a specific polyclonal anti-asprosin antibody. (A) Domain structure of fibrillin-1 and its N- and C-terminal halves. The C-terminal propeptide sequence of fibrillin-1 after furin cleavage (furin cleavage site marked by arrow and dashed line, furin cleavage sequence underlined) yields asprosin (marked in grey). The 140 amino acids representing human asprosin S2732-H2871 were recombinantly produced in HEK293 cells. (B) Coomassie stained quality control gel of recombinantly produced and purified (> 95% purity) human asprosin. Asprosin was overexpressed with a C-terminally placed 2 × -Strep-tag II, and eluted fractions (F2-F6) after affinity chromatography were subjected to reducing SDS-PAGE using a 12% gel. (C) Mass spectrometry analysis revealed that all of the predicted three N-linked glycosylation sites (marked in red, position regarding the fibrillin-1 sequence in parentheses) are used as indicated by the + 1 mass shift expected for de-N-glycosylated peptides (see also Supplementary Table S2). Underlined residues mark predicted cleavage sites of V8 and trypsin used to generate peptides of de-N-glycosylated asprosin. No unequivocal assignments of O-glycosylation sites were possible on the basis of peptide masses in MS1 spectra. Residues representing linker regions are indicated in green, thrombin cleavage (LVPRGS) site is underlined, and Strep-tag II sequences are marked in blue. (D) Removal of N-linked, as well as many common O-linked glycans, after incubation in a deglycosylation mix results in significant loss of molecular weight of human asprosin. (E) (left) Polyclonal anti-asprosin serum (pc-asp anti-asprosin antibody) raised in rabbit showed a high titer in ELISA assay detecting coated asprosin (100 ng/well). Half maximum signal was already reached at a 1:10,000 dilution. Rabbit pre-serum (before rabbit immunization) served as a control. Recombinant asprosin was coated in duplicates (100 ng/well). (right) ELISA showing high specificity of the pc-asp antibody. No cross-reactivity to the N-terminal region of fibrillin-1 (rF90), C-terminal region of fibrillin-1 (rF6), human albumin, or human IgG was detected. Data points represent mean ± SD of duplicates. (F) (left) Pc-asp antibody specifically recognizes human asprosin in western blot analysis, with no observable cross-reactivity to the N-terminal region (rF90) and C-terminal region (rF6) of fibrillin-1. (middle) Detection of N-terminal fibrillin-1 (rF90) with labmade fibrillin-1 rF90 antibody. (right) Specific detection of the C-terminal fibrillin-1 (rF6) with CPTC-FBN1-3 antibody (DSHB, Iowa, USA) which was raised against a synthetic peptide in the rF6 region. (G) Reduced signal of deglycosylated asprosin in western blot by pc-asp anti-asprosin antibody. Data were analyzed using Graphpad Prism version 8.0.2.