Abstract
Background
The BNT162b2 vaccine received emergency use authorization from the U.S. Food and Drug Administration for the prevention of severe coronavirus disease 2019 (COVID-19) infection. We report a case of biopsy and magnetic resonance imaging (MRI)-proven severe myocarditis that developed in a previously healthy individual within days of receiving the first dose of the BNT162b2 COVID-19 vaccine.
Case Summary
An 80-year-old female with no significant cardiac history presented with cardiogenic shock and biopsy-proven fulminant myocarditis within 12 days of receiving the BNT162b2 COVID-19 vaccine. She required temporary mechanical circulatory support, inotropic agents, and high-dose steroids for stabilization and management. Ultimately, her cardiac function recovered, and she was discharged in stable condition after 2 weeks of hospitalization. A repeat cardiac MRI 3 months after her initial presentation demonstrated stable biventricular function and continued improvement in myocardial inflammation.
Discussion
Fulminant myocarditis is a rare complication of vaccination. Clinicians should stay vigilant to recognize this rare, but potentially deadly complication. Due to the high morbidity and mortality associated with COVID-19 infection, the clinical benefits of the BNT162b2 vaccine greatly outweighs the risks of complications.
Keywords: Cardiogenic shock, COVID-19, Vaccine, Myocarditis, Mechanical circulatory support device, Case report
Learning points.
Early recognition of fulminant myocarditis is critical in improving survival and initiation of appropriate therapy
Temporary mechanical circulatory support devices may play a critical role in recovery of patients with cardiogenic shock from myocarditis
Vaccines may provoke myocarditis in very rare instances. However, benefits of vaccination greatly outweigh the risks
Cardiology, infectious disease, pulmonary and critical care, pathology
Specialties other than cardiology involved
Cardiology, infectious disease, pulmonary and critical care, pathology
Introduction
Emergency use authorization for the BNT162b2 vaccine to prevent coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was approved by the US Food and Drug Administration (FDA) on 11 December 2020 for individuals 16 years or older.1 Two doses of the BNT162b2 vaccine provided a 95% protection against COVID-19 and demonstrated a similar safety profile compared to other viral vaccines over a median period of 2 months.2 Despite the widespread administration of the vaccine, limited data exist on rare cardiac side effects from its use.
Timeline
| Date | Significant event |
|---|---|
| 2 February 2021 | Patient received her first dose of the BNT162b2 coronavirus disease 2019 vaccine. |
| 14 February 2021 | She presented to the emergency room with symptoms of nausea, emesis, and diarrhoea. |
| 15 February 2021 | Inotropes were started due to worsening cardiogenic shock. |
| 17 February 2021 | She continued to decompensate despite maximal pressor support. Impella CP placed. She was transferred to a tertiary centre and intubated on arrival. |
| 20 February 2021 | She was extubated. |
| 21 February 2021 | Impella CP was removed |
| 26 February 2021 | She was discharged in stable condition and with oral medications for her heart failure. |
| 26 May 2021 | She presented to clinic for follow-up and repeat cardiac magnetic resonance imaging has been completed showing normal biventricular function. |
Case presentation
An 80-year-old Caucasian female presented to a local hospital with 4 days of progressive emesis, generalized abdominal discomfort, and diarrhoea. She had no known COVID-19 exposure or infection in the past and received the first dose of the BNT162b2 vaccine 7 days prior to symptom onset. She reported no immediate symptoms following vaccination.
She had a prior appendectomy, cholecystectomy, and hysterectomy. Stress echocardiogram obtained in 2015 for atypical chest pain was negative for ischaemia and showed normal left ventricular (LV) function. Her medications included ranitidine, cholecalciferol, and daily fish oil capsules and had no significant alcohol, smoking, or illicit drug use.
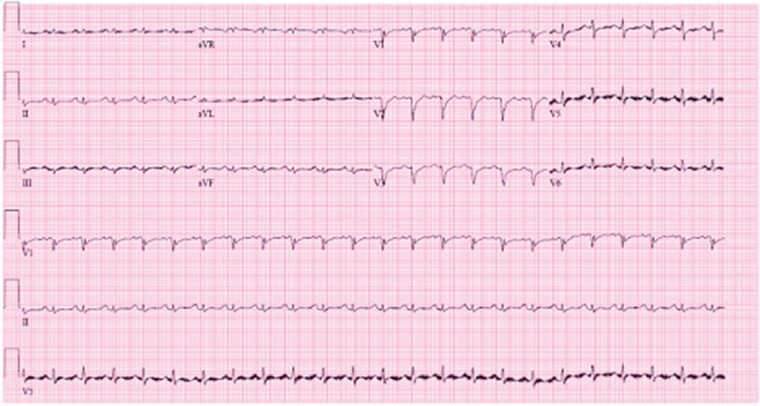
Upon presentation, she was afebrile with a heart rate of 98 beats per minute, arterial oxygen saturation of 99% on room air, and blood pressure of 94/64 mmHg. Electrocardiogram (ECG) showed non-specific ST-segment and T-wave abnormalities and low voltage QRS complexes throughout (similar to that obtained at the University of Minnesota, shown in Figure 1). Initial laboratory findings are presented in Table 1. Troponin I was critically elevated at 72.65 ng/mL, B-type natriuretic peptide was 1511 pg/mL, and reverse transcription polymerase chain reaction (RT-PCR) was negative for SARS-CoV-2.
Figure 1.
Electrocardiogram obtained following patient transfer. Electrocardiogram shows sinus tachycardia with non-specific ST-segment and T-wave changes and diffuse low voltage QRS complexes.
Table 1.
Laboratory values at initial presentation
| Variable | Value on admission | Normal range |
|---|---|---|
| WBC count (thousand/mm3) | 5.2 | 4.5–11 |
| RBC count (million/mm3) | 4.55 | 4–5.2 |
| Platelet count (thousand/mm3) | 158 | 140–440 |
| Sodium (mmol/L) | 133 | 135–145 |
| Serum creatinine (mg/dL) | 1.15 | 0.57–1.11 |
| Troponin I (ng/mL) | 72.65 | <0.034 |
| BNP (pg/mL) | 1511 | <265 |
| Albumin (g/dL) | 4.1 | 3.2–4.6 |
| AST (IU/L) | 323 | 2–40 |
| ALT (IU/L) | 167 | 8–45 |
| Total bilirubin (mg/dL) | 0.6 | 0.2–1.2 |
| SARs-CoV-2 PCR | Negative | Negative |
| Influenza A/B PCR | Negative | Negative |
| C-reactive protein (mg/L) | 37 | <8 |
| Procalcitonin (ng/mL) | 0.16 | 0.5 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BNP, B-type natriuretic peptide; RBC, red blood cell; WBC, white blood cell.
Computed tomography of the abdomen and pelvis obtained to further evaluate her gastrointestinal symptoms showed evidence of anasarca but was otherwise unremarkable. Coronary angiogram revealed non-occlusive coronary artery disease. Subsequent transthoracic echocardiogram (TTE) showed normal LV size, moderate LV hypertrophy, and LV ejection fraction of 35% with moderate global hypokinesis.
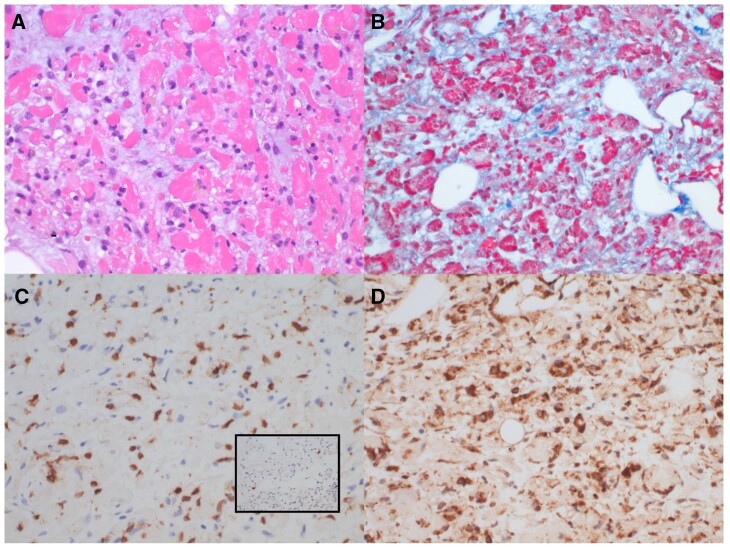
The following day, she had increasing oxygen requirement and developed progressive hypotension. Troponin I was down-trending, but C reactive protein peaked at 120 mg/L. Right heart catheterization revealed a right atrial pressure of 11 mmHg, pulmonary artery pressure of 45/32 (36) mmHg, and pulmonary capillary wedge pressure of 32 mmHg. Cardiac index and systemic vascular resistance were calculated at 1.4 L/min/m2 (Fick) and 2400 dynes.s.cm–5, respectively. Endomyocardial biopsy (EMB) was obtained. Given evidence of cardiogenic shock, dobutamine, nitroprusside, and bumetanide drips were started. Despite these interventions, her oxygen requirement continued to increase, creatinine was elevated at 2.05 mg/dL, and lactic acid level rose to 5.8 mmol/L. Impella CP (Abiomed Inc., Danvers, MA, USA) was placed for systemic hypoperfusion and worsening end-organ function. Left ventricular ejection fraction was 20% post-procedure. Endomyocardial biopsy revealed fulminant myocarditis with extensive myocyte damage out of proportion to the inflammatory infiltrates. No giant cells or granulomas were revealed (Figure 2). Her haemodynamic and respiratory status continued to worsen, prompting the administration of 1000 mg methylprednisolone and transfer to our institution.
Figure 2.
Right ventricular endomyocardial biopsy. H&E-stained sections show marked, predominating myocyte damage and endomysial oedema, with patchy, mild-moderate inflammatory infiltrates consisting of macrophages, lymphocytes, eosinophils, and scattered plasma cells. No giant cells were observed. (A) Trichrome stain highlights myocyte damage and oedema. (B) Immunohistochemical stain for CD3 shows scattered T-cells (C), while immunohistochemical stain for CD20 shows only rare B-cells (C, inset). Immunohistochemical stain for CD68 confirms frequent macrophages within the endomysium (D). CD138 staining showed rare plasma cells, studies for cytomegalovirus (CMV) and in situ hybridization for Epstein–Barr Virus were negative (not shown).
Upon arrival, her mean arterial pressure was 72 mmHg with the Impella CP set at P7. She remained somnolent prompting endotracheal intubation. Amiodarone drip was started due to frequent episodes of paroxysmal atrial flutter and non-sustained ventricular tachycardia associated with haemodynamic instability. She was continued on methylprednisolone 1000 mg per day for 3 days followed by rapid prednisone taper. A thorough infectious workup was completed with negative blood, sputum and urine cultures. Repeat RT-PCR for SARS-CoV-2 was negative but spike receptor binding domain antibody titre was positive at 1:6400. Other viral studies including Influenza A (including H1 and H1N1) and B, Parainfluenza Virus, Coronavirus, Human Metapneumovirus, Rhinovirus/Enterovirus, Respiratory Syncytial Virus, Coxsackie Virus, Adenovirus, Parvovirus B19, Cytomegalovirus, Hepatitis C, Human Immunodeficiency Virus, Chlamydia pneumoniae, and Mycoplasma pneumoniae were also negative. Epstein–Barr Virus DNA was positive at 1417 copies/mL and this was thought to be consistent with virus reactivation. D-Dimer was elevated at 3.4 μg/mL and interleukin-6 (IL-6) peaked at 93.4 pg/mL.
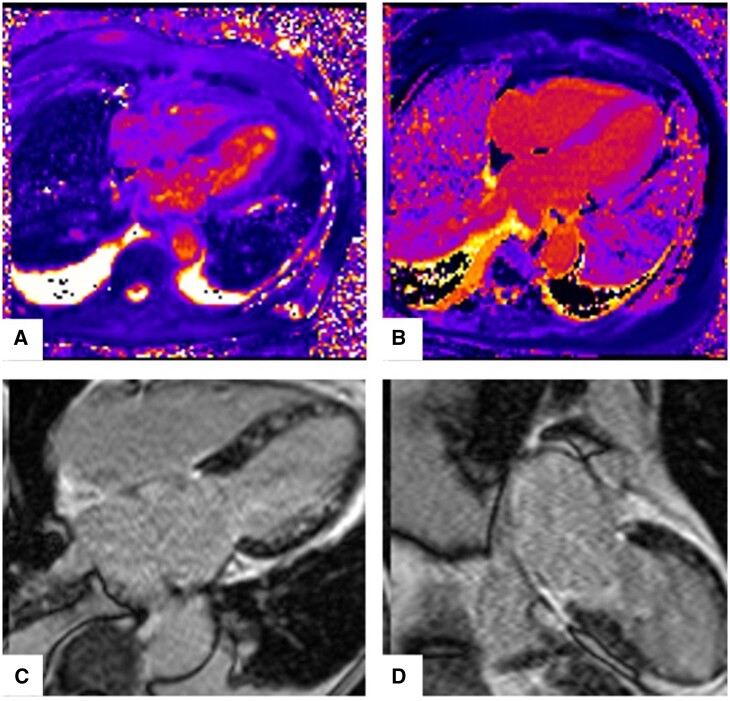
On Day 7, Impella device was removed, and she was extubated. Repeat TTE on Day 8 showed normal LV function with mild to moderately reduced right ventricular function. Dobutamine was weaned off as cardiac index increased to 3.1 L/min/m2 with oral afterload reduction and renal function recovered to baseline. Cardiac magnetic resonance imaging (MRI) was notable for normal biventricular function and acute myocarditis (Figure 3). She was discharged in stable condition and on the following medications for heart failure (HF) management: metoprolol succinate 25 mg daily and spironolactone 25 mg daily.
Figure 3.
Cardiac magnetic resonance imaging at presentation showing acute myocarditis. (A) Diffuse myocardial oedema on T2 mapping. Mean T2 = 71 ms. (B) Diffuse mid-myocardial fibrosis on T1 mapping. (C, D) Diffuse patchy mid-myocardial late gadolinium enhancement (LGE).
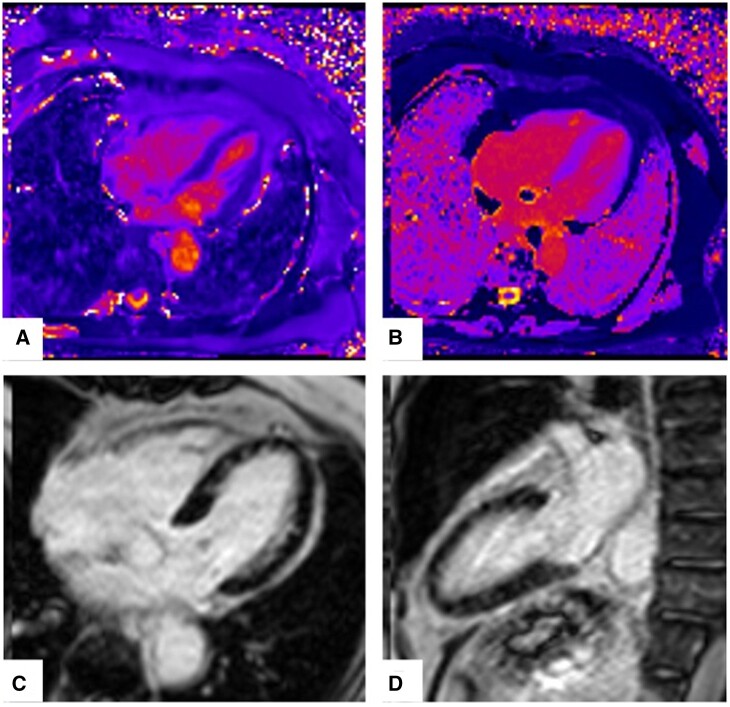
We followed her very closely in our outpatient HF clinic. Her overall condition continued to improve, laboratory values remained normal, and HF medications were titrated. Repeat cardiac MRI at 3 months showed resolving myocarditis with normal biventricular function, persistent myocardial oedema, and significantly reduced burden of patchy mid-myocardial fibrosis in the LV (Figure 4). At 6 months follow-up, she continued to do well and regained her prior-to-admission functional status.
Figure 4.
Follow-up cardiac magnetic resonance imaging at 3 months with resolving myocarditis. (A) Diffuse myocardial oedema on T2 mapping. Mean T2 = 51 ms. (B) Diffuse mid-myocardial fibrosis on T1 mapping. (C, D) Residual patchy mid-myocardial LGE.
Discussion
We present a case of fulminant myocarditis in a previously healthy 80-year-old female, days after receiving the first dose of the BNT162b2 COVID-19 vaccine.
Fulminant myocarditis is a rare condition characterized by acute, severe diffuse inflammation of the myocardium often leading to cardiogenic shock, life-threatening ventricular arrhythmias, multi-organ failure, and death. Major histologic subtypes include giant cell, lymphocytic, and eosinophilic myocarditis.3 The most common aetiologies are viral infections particularly coxsackievirus and adenovirus, autoimmune disorders, toxic substances, and medications. Relevant to our patient, the extensive workup was negative. She had no history of autoimmune disorders and had documented negative results for thyroid peroxidase antibody, anti-cyclic citrullinated peptide antibody, rheumatoid factor, and antinuclear antibodies making new-onset autoimmune disease unlikely. Giant cells were not seen on the EMB, but it is not possible to completely exclude this aetiology. Although the yield of initial RV biopsy approaches 80%, a repeat biopsy may be necessary to reduce sampling error.4 We did not pursue a second biopsy initially due to patient’s haemodynamic instability. Later, it was deferred given the patient’s atypical age for giant cell myocarditis and profound clinical response to steroid therapy alone. Although a direct causal relationship cannot be demonstrated between the first BNT162b2 COVID-19 vaccine dose and our patient’s fulminant myocarditis, the meticulous exclusion of other aetiologies and the timing between the events render the relationship plausible. It is important to emphasize that we are not able to completely exclude that our patient had a prior, silent COVID-19 infection that may have increased her risk for vaccine-associated myocarditis. However, our patient practiced maximum social distancing, had no known exposures and no family members had history of positive COVID-19 test. With this information, we believe that prior infection was unlikely.
Prior reports have linked myocarditis to different vaccinations, including influenza and smallpox.5–8 The underlying aetiology is thought to be non-specific inflammatory process vs. provoked autoimmune response in the setting of molecular mimicry.7 Although causal relationship cannot be demonstrated between the first BNT162b2 COVID-19 vaccine dose and our patient’s fulminant myocarditis, the meticulous exclusion of other aetiologies and the timing between the events render the relationship plausible.
The BNT162b2 COVID-19 vaccine is one of two mRNA vaccines available in the market.2,9 It is a lipid nanoparticle-formulated, nucleoside-modified RNA (mRNA) encoding the SARS-CoV-2 full-length spike, modified by two proline mutations to lock it in the prefusion conformation.2 The lipid particles allows for transfer of the RNA into host cells, resulting in the SARS-CoV-2 S antigens’ expression, which then generates immunogenicity and antibody response that confers protection to COVID-19.10
Several peer-reviewed case reports described acute myocarditis following COVID-19 vaccination.11,12 Data from the Advisory Committee on Immunization Practices (ACIP) reported that majority of these cases occur in males, <30 years of age. Presenting signs and symptoms include chest pain, ST- or T-wave changes on ECG, elevated cardiac enzymes, and abnormal cardiac imaging. Time to onset of symptoms was a median of 3 days after vaccination and usually after the second dose.13
Despite these published reports, a causal relationship has not be established.11,12,14 Proposed mechanisms for myocarditis include molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens, triggering of pre-existing dysregulated immune pathways, immune response to mRNA, activation of immunologic pathways, and dysregulated cytokine expression.14 Male predominance of vaccine-related myocarditis is thought to be due testosterone’s role in the inhibition of anti-inflammatory cells as well as possibly to underdiagnosis of this disease process in women.14
Management of COVID-19 myocarditis is mainly supportive. These include non-steroidal anti-inflammatory drugs, steroids, colchicine, intravenous immunoglobulin, and guideline-directed medical therapy for those with systolic heart failure. Early referral to cardiology and infectious disease specialists is recommended to ensure appropriate follow-up and guidance on subsequent immunization strategies.14 Majority of patients recover without any significant complications. Pooled data from published case reports demonstrated that COVID-19 vaccine-related myocarditis have resolution of clinical symptoms within 6 days with preservation of cardiac function.12
The case we presented is unique as there have only been 10 cases reported of COVID-19 myocarditis among individuals ≥65 years of age.14 This highlights that clinicians should have high index of suspicion for vaccine-related myocarditis even in the older population.
Given the high morbidity and mortality associated with COVID-19 infection, the benefit of vaccination greatly outweighs the risks. The ACIP continues to recommend using mRNA COVID-19 vaccines despite the risks for myocarditis in all populations, including adolescents and young adults.15 However, providers should stay vigilant to recognize signs of myocarditis enabling early diagnosis and prompt management.
Lead author biography

Arianne Agdamag, MD is a current third-year General Cardiology fellow at the University of Minnesota. She obtained her medical degree from the University of the Philippines and completed her Internal Medicine residency at Rush University Medical Center in Chicago, Illinois. She has a special interest in advanced heart failure and heart transplantation.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that witnessed verbal consent for submission and publication of this case report including images and associated text has been obtained from the patients detailed in this case report. This has been discussed with the editors.
Conflict of interest: None declared
Funding: None declared.
Supplementary Material
References
- 1. FDA. Pfizer-BioNTech COVID-19 Vaccine. 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (5 August 2021).
- 2. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. ; American Heart Association Heart Failure and Transplantation Committee of the Council on Clinical Cardiology. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation 2020;141:e69–e92. [DOI] [PubMed] [Google Scholar]
- 4. Shields RC, Tazelaar HD, Berry GJ, Cooper LT Jr.. The role of right ventricular endomyocardial biopsy for idiopathic giant cell myocarditis. J Card Fail 2002;8:74–78. [DOI] [PubMed] [Google Scholar]
- 5. Engler RJ, Nelson MR, Collins LC Jr, Spooner C, Hemann BA, Gibbs BT. et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One 2015;10:e0118283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nagano N, Yano T, Fujita Y, Koyama M, Hasegawa R, Nakata J. et al. Hemodynamic collapse after influenza vaccination: a vaccine-induced fulminant myocarditis? Can J Cardiol 2020;36:1554 e5–e7. [DOI] [PubMed] [Google Scholar]
- 7. Keinath K, Church T, Kurth B, Hulten E.. Myocarditis secondary to smallpox vaccination. BMJ Case Rep 2018;2018:bcr2017223523. doi: 10.1136/bcr-2017-223523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E.. Myocarditis and pericarditis after immunization: gaining insights through the vaccine adverse event reporting system. Int J Cardiol 2018;273:183–186. [DOI] [PubMed] [Google Scholar]
- 9. El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ. et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med 2021;385:1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaur SP, Gupta V.. COVID-19 vaccine: a comprehensive status report. Virus Res 2020;288:198114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L. et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 2021;6:1202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salah HM, Mehta JL.. COVID-19 vaccine and myocarditis. Am J Cardiol 2021;157:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CDC CfDC. Advisory Committee on Immunization Practices (ACIP). Coronarvirus disease 2019 (COVID-19) vaccines. https://www.cdc.gov/vaccines/acip/meetings/slides-2021-06.html (5 August 2021).
- 14. Bozkurt B, Kamat I, Hotez PJ.. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME. et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the Advisory Committee on Immunization Practices - United States, June 2021. Morb Mortal Wkly Rep 2021;70:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.