Abstract
BACKGROUND
Heterogeneous macrophages play an important role in multiple liver diseases, including viral fulminant hepatitis (VFH). Fibrinogen-like protein 2 (FGL2) is expressed on macrophages and regulates VFH pathogenesis; however, the underlying mechanism remains unclear.
AIM
To explore how FGL2 regulates macrophage function and subsequent liver injury during VFH.
METHODS
Murine hepatitis virus strain 3 (MHV-3) was used to induce VFH in FGL2-deficient (Fgl2-/-) and wild-type (WT) mice. The dynamic constitution of hepatic macrophages was examined. Adoptive transfer of Fgl2-/- or WT bone marrow-derived macrophages (BMDMs) into WT recipients with macrophages depleted prior to infection was carried out and the consequent degree of liver damage was compared. The signaling cascades that may be regulated by FGL2 were detected in macrophages.
RESULTS
Following MHV-3 infection, hepatic macrophages were largely replenished by proinflammatory monocyte-derived macrophages (MoMFs), which expressed high levels of FGL2. In Fgl2-/- mice, the number of infiltrating inflammatory MoMFs was reduced compared with that in WT mice after viral infection. Macrophage depletion ameliorated liver damage in WT mice and further alleviated liver damage in Fgl2-/- mice. Adoptive transfer of Fgl2-/- BMDMs into macrophage-removed recipients significantly reduced the degree of liver damage. Inhibition of monocyte infiltration also significantly ameliorated liver damage. Functionally, Fgl2 deletion impaired macrophage phagocytosis and the antigen presentation potential and attenuated the proinflammatory phenotype. At the molecular level, FGL2 deficiency impaired IRF3, IRF7, and p38 phosphorylation, along with NF-κB activation in BMDMs in response to viral infection.
CONCLUSION
Infiltrated MoMFs represent a major source of hepatic inflammation during VFH progression, and FGL2 expression on MoMFs maintains the proinflammatory phenotype via p38-dependent positive feedback, contributing to VFH pathogenesis.
Keywords: Viral fulminant hepatitis, Fibrinogen-like protein 2, Proinflammatory macrophages, Infiltrating macrophages, P38
Core Tip: In this study, we demonstrate that: (1) Monocytes infiltrating the liver represent a major source of hepatic inflammation, which has a decisive effect on the pathogenesis of viral fulminant hepatitis; (2) Viral infection induces FGL2 expression on macrophages, which is required for maintaining the inflammatory phenotype and cell function; and (3) FGL2 generates a positive feedback loop of an inflammatory cascade in macrophages in response to viral infection.
INTRODUCTION
Viral fulminant hepatitis (VFH) is a type of acute liver failure (ALF) that can develop after viral infection in patients with hepatitis A, hepatitis B, and hepatitis E. VFH is a devastating syndrome characterized by severe liver injury with coagulation abnormalities and hepatic encephalopathy[1]. Pathologically, acute liver injury and subsequent massive hepatocyte loss are caused by hyperactivation of the innate immune response, followed by an excessive adaptive immune response to viral infection, leading to systemic inflammation. Innate immune cells, activated by pathogen-associated molecular patterns (PAMPs) from invading pathogens, damage-associated molecular patterns (DAMPs) released from necrotic cells, and afferent endotoxins from the portal vein, are dominant in conditions of sterile inflammation and non-virus-associated ALF[2,3]. Adaptive immunity activated by PAMPs seems to play a more important role in VFH than innate immunity because of the exposure to abundant viral antigens presented by antigen-presenting cells[4,5]. However, the extent of the contribution of innate immunity in generating viral antigens or DAMPs in VFH is not well defined.
Liver-resident macrophages, also known as Kupffer cells (KCs), are the largest population of hepatic immune cells that play an essential role in maintaining liver homeostasis and ensure rapid responses to hepatic insults[6]. Monocyte-derived macrophages (MoMFs) are an ontologically distinct subpopulation of hepatic macrophages, which are found in only small numbers under physiological conditions and then serve to replenish the macrophage population during liver injury[7]. Macrophages are generally classified into proinflammatory (M1) and anti-inflammatory (M2) macrophages (or alternatively activated macrophages), phenotypically corresponding to T helper (Th)1 and Th2 responses, respectively. However, the spectrum of macrophage classification remains obscure as the transition between macrophage subtypes occurs in response to microenvironment mediators[8,9]. Dynamic alterations in KC and MoMF populations were observed in the context of sterile inflammation in an experimental model of ALF induced by acetaminophen overdose, with replacement of KCs by MoMFs during disease progression[10]. Further analyses of this model revealed differential subsets of macrophages showing the protective and detrimental characteristics of KCs and MoMFs during the course of acetaminophen-induced ALF[10,11]. Moreover, an increase in the number of MoMFs with potent activity has been observed in non-pregnant patients with hepatitis E-associated ALF compared with that in pregnant patients. In a model of experimental VFH, hepatic leukocyte numbers were found to increase at an early stage of liver injury[12], suggesting the involvement of macrophages in disease progression. However, further investigation is needed to elucidate the precise roles of the distinct subpopulations of hepatic macrophages in VFH.
Fibrinogen-like protein 2 (FGL2), a membrane protein with prothrombinase at the N-terminal, is expressed in a variety of cells, such as macrophages, dendritic cells, and endothelial cells, and can be induced robustly and exclusively in macrophages in response to stimulation with cytokines [interferon (IFN)-gamma or tumor necrosis factor (TNF)-α), viral infection, and lipopolysaccharide (LPS)[7,13]. This suggests that FGL2 itself is a critical mediator of inflammation in that the interaction between inflammation and coagulation is reciprocally exacerbated in terms of inflammatory cytokine production and tissue factor secretion[11]. FGL2 deficiency was shown to prevent the development of VFH in mice following experimental murine hepatitis virus strain 3 (MHV-3) infection; this effect was speculated to be mainly dependent on its ability to regulate procoagulant activity, as evidenced by concomitant expression of FGL2 and fibrinogen production[14]. Emerging evidence suggests that FGL2 is a downstream effector molecule in the inflammatory cascades associated with VFH development and progression, which involve C5aR, TNF-α, and MSR1[7,12,15,16]. Interestingly, complement activation, a critical step for inflammation initiation, has also been observed in FGL2-deficient mice following MHV-3 infection[16], highlighting a complex regulatory network of inflammation involving FGL2 and other effectors. Therefore, we hypothesized that FGL2 plays a role in the reconstitution of hepatic macrophages to regulate proinflammatory macrophage activation that contributes to VFH progression and exacerbation. To test this hypothesis, we first explored the hepatic macrophage composition and expression pattern of FGL2 during the progression of VFH in patients with and without hepatitis B virus (HBV)-associated acute-on-chronic liver failure (ACLF), since the pathology of ACLF closely resembles that of ALF[17]. To explore the role of FGL2 in VFH progression and the underlying mechanism, we determined alterations in hepatic macrophage populations during progression of VFH in FGL2-deficient (Fgl2-/-) and wild-type (WT) mice intraperitoneally injected with MHV-3 at different stages after infection.
MATERIALS AND METHODS
Ethics statement
All studies on human subjects were approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (permit number: TJ-C20170924), and all participants provided informed consent in compliance with the Helsinki Declaration revised in 1983.
The animal experiments were approved by the Institutional Animal Care and Use Committee of Tongji Hospital (permit number: TJ-A20171008) and were conducted in accordance with state guidelines from the Ministry of Science and Technology of China.
Patient samples
Seven patients with ACLF, as defined by the Asian Pacific Association for Study of the Liver (APASL) guidelines[18], who were undergoing liver transplantation were included in the study; liver samples were obtained during the surgery. The clinical features of the patients are summarized in Table 1. Control samples (n = 3) were obtained from a healthy liver donor and from the paratumor tissues of a patient with hepatic hemangioma and a patient with hepatocellular carcinoma without cirrhosis.
Table 1.
Clinical characteristics of patients and controls
| Characteristics | ACLF (n = 7) | Healthy controls (n = 3) |
| Age (year) | 47.14 ± 3.3a | 30.33 ± 3.4 |
| Sex (male:female) | 6:1 | 3:0 |
| Laboratory evaluation | ||
| ALT (U/L) | 642.9 ± 360.3 | 8.67 ± 1.76 |
| Tbil (μmol/L) | 429.8 ± 70.53a | 17 ± 2.34 |
| PTA (%) | 30.29 ± 3.3a | 118 ± 12.17 |
| HBsAg (ng/mL) | 3870 ± 1600 | 0 |
| HBV-DNA (106 copies/mL) | 8.18 ± 6.78 | 0 |
| Ascites and/or Encephalopathy | 7 | 0 |
P < 0.5 vs controls.
Age, Serum alanine aminotransferase, total bilirubin, prothrombin time activity shown as mean ± SD. ACLF: Acute on chronic liver failure; ALT: Alanine aminotransferase; Tbil: Total bilirubin; PTA: Prothrombin time activity.
Animals, viral infection, macrophage depletion, and inhibitor treatment
Female BALB/c mice (HFK Bioscience Company Ltd., Beijing, China) at 6-8 weeks of age were used in all animal experiments. The mice were housed in a controlled environment (specific pathogen-free, 12 h light/dark cycle, 21 ± 2 °C, humidity 50 ± 10%) and had free access to food and water. Fgl2-/- mice were generated by introducing a deletion at the N-terminal of the open reading frame of the Fgl2 locus using CRISPR/Cas9 technology. WT BALB/c mice were used as controls. To establish the fulminant hepatitis model, 100 plaque-forming unit (PFUs) of MHV-3 (American Type Culture Collection, Manassas, VA, USA) dissolved in 200 µL saline was injected into the WT and Fgl2-/- mice intraperitoneally. To deplete macrophages, mice were intravenously injected with 200 µL clodronate liposomes or phosphate-buffered saline (PBS)-liposomes as a control (Liposome B.V., the Netherlands) 24 h prior to MHV-3 injection. To inhibit MoMF infiltration, a chemokine receptor-2 (CCR2) inhibitor (cenicriviroc; 10 mg/kg) was intraperitoneally injected 24 h prior to MHV-3 infection. WT or Fgl2-/- mice were randomly selected to be sacrificed at 0, 24, 48, and 72 h following MHV-3 injection, and liver and blood samples were harvested for analysis.
Cell isolation
Hepatic mononuclear non-parenchymal cells from WT and Fgl2-/- mice were isolated via liver perfusion, enzymatic digestion, and differential centrifugation, as described previously[19]. Peritoneal exudate macrophages (PEMs) were isolated from the mice 3 d after intraperitoneal injection of 3% starch broth (1 mL/mice). Bone marrow-derived macrophages (BMDMs) were differentiated from hematopoietic stem cells isolated from the femurs cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and macrophage-colony stimulating factor (30 μg/mL, Peprotech) for 7 days. BMDMs or PEMs were maintained in DMEM supplemented with 10% FBS and treated with LPS (100 ng/mL), interleukin (IL)-4 (20 ng/mL), or MHV-3 (5 × 105 PFU/mL).
Macrophage adoptive transfer
KCs are the largest population of hepatic resident non-parenchymal cells and the first line of defense against pathogens and toxins[6]. The priming of KCs may therefore be involved in initiation of the hepatic immune response, a critical step for subsequent exacerbation of inflammatory accumulation. Accordingly, we also assessed liver damage following MHV-3 infection with depletion of macrophages by clodronate liposomes. WT mice were treated with 200 µL clodronate liposomes or PBS-liposomes as a control to deplete macrophages, which then served as the recipient mice for adoptive transfer. Harvested BMDMs from WT and Fgl2-/- mice were intravenously injected into the recipient mice at 5 × 105 cells/mouse. MHV-3 (100 PFU/mouse) injection was performed 24 h later. Liver and blood samples were harvested at 48 h post MHV-3 infection for further analyses.
Flow cytometry
Single-cell suspensions were blocked with 2 μg/mL anti-CD16/CD32, followed by staining with fluorescence-conjugated antibody cocktails. For intracellular staining, surface-stained cells were fixed and permeabilized using Cytofix/Cytoperm Fixation/Permeabilization Kit (No. 554714, BD Pharmingen) prior to incubation with target antibodies. Data were acquired on a BD FACS Canto II flow cytometer (BD Bioscience, San Jose, CA) and analyzed using FACS Diva software.
Detailed procedures and other materials are described in the supporting information.
Statistical analyses
Statistical testing was performed using GraphPad Prism 5.0 software (GraphPad Software Inc., San Diego, CA, United States). Comparisons between WT and Fgl2−/− mice (or other situations where only two groups are compared) were performed using Student’s unpaired t-test; one-way analysis of variance with Tukey’s post-hoc test or Mann-Whitney U test was used for multiple comparisons (unless otherwise indicated). Data are presented as mean ± standard deviation; P < 0.05 was considered to indicate a statistically significant difference.
RESULTS
The number of proinflammatory macrophages with elevated FGL2 expression increases in patients with hepatitis virus-induced liver failure
Marked infiltration of leukocytes was observed in samples of the patients with ACLF, along with massive necrosis in the parenchymal lobules (Figure 1A). CD68+ macrophages markedly accumulated in the periportal areas (Figure 1B, Supplementary Figure 1), suggesting widespread liver damage[20]. Furthermore, macrophages were polarized to proinflammatory macrophages, as confirmed by the expression of S100A9 (Figure 1C), an alarmin molecule expressed in proinflammatory macrophages, activated neutrophils, and granulocytes, which is often used as a biomarker of inflammation[21]. Interestingly, the majority of S100A-positive activated proinflammatory macrophages also expressed FGL2 in consecutive sections of liver samples from patients with HBV-related liver failure (Figure 1D). These data suggest that a high expression level of FGL2 could be an important molecular event of hepatic macrophage polarization during viral liver failure.
Figure 1.
Fibrinogen-like protein 2 was expressed on proinflammatory macrophages in patients with hepatitis B virus-associated liver failure. A: Representative image of liver sections (200×) subjected to Hematoxylin and eosin staining (H&E); Black circles point to transparent necrosis, red circles point to infiltrating leukocytes; B: Calculation of CD68+ macrophages at periportal and lobular areas; C: Immunofluorescence against CD68, S100A9 on liver sections (200×) from patients of hepatitis B virus-associated acute chronic-on liver failure (acute-on-chronic liver failure (ACLF), n = 7) and healthy controls (n = 3); D: Immunofluorescence against S100A9 and fibrinogen-like protein 2 on liver sections (200×) from ACLF patients (n = 7) and healthy controls (n = 3), representative images on the left and statistical columns on the right. Data are expressed as mean ± SD.
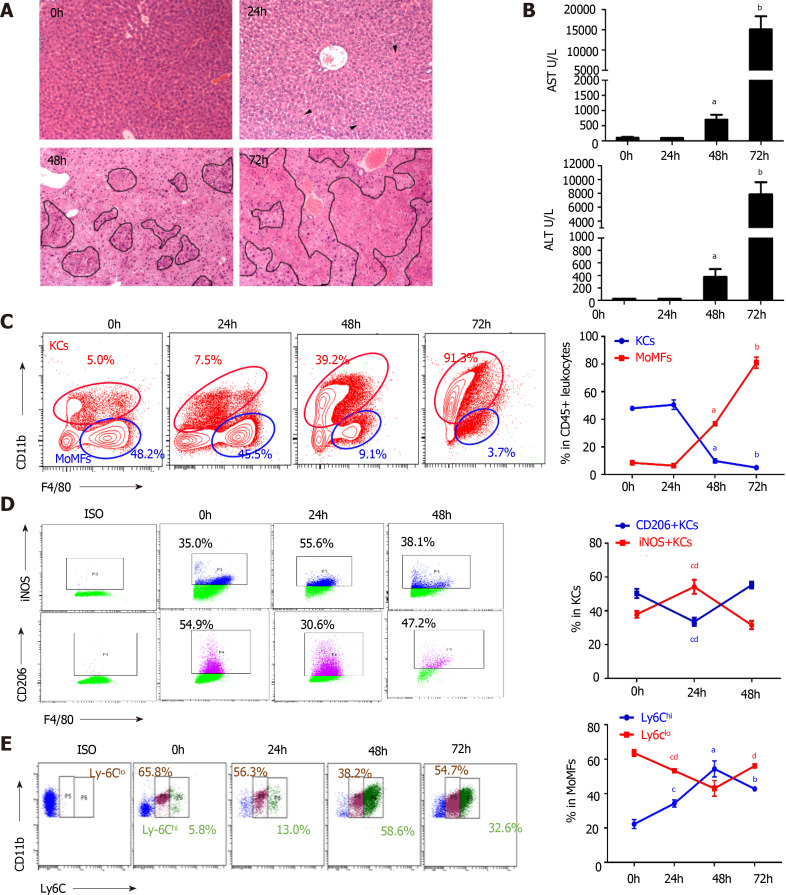
Ly6Chigh MoMFs dominate the hepatic macrophage population during VFH progression
In the experimental fulminant hepatitis mouse model, pathogenesis was divided into an early stage without global liver damage at 24 h post-infection of MHV-3, a progression phase with continuous enlargement of block necrosis and increased transaminase levels, and an end stage defined at 72 h post-infection when the animals were near death (Figure 2A and B). At the early stage of infection, hematoxylin and eosin staining showed that the hepatic architecture was intact and no obvious macrophages infiltration was noted (Figure 2A). Aggregating plots of the CD11b F4/80-expressing flow cytometry panel[22] showed a continuous increase in the number of hepatic MoMFs (CD11bhigh F4/80int) and a decline in the number of KCs (CD11blow F4/80high) over the course of infection, accompanied by enlarged lesions until confluent necrosis of the liver lobules occurred at the later stage (Figure 2A and C). Notably, KCs constituted the dominant hepatic macrophage population at the early stage of infection, with an increased M1 macrophage subset [induced nitric oxide synthase (iNOS+) KCs] (Figure 2D, Supplementary Figure 2A and C). However, MoMFs subsequently dominated the hepatic macrophage population, characterized by an increased ratio of Ly6Chigh cells; however, the Ly6Chigh/Ly6Clow ratio decreased at the end stage of the disease (Figure 2E, Supplementary Figure 2A), implying a transition from Ly6Chigh MoMFs to Ly6Clow MoMFs. The dynamic alterations in hepatic macrophage populations were also revealed by an increase in the levels of proinflammatory cytokines TNF-α and IL-6 during progression, which then decreased at the end stage, along with a continued decrease in transforming growth factor (TGF)-β levels in macrophages (Supplementary Figure 2D). Consistent with the reconstitution of hepatic macrophage populations in which Ly6Chigh MoMFs are more inflammatory with an expanded spectrum of cytokine and chemokine expression compared with M1 macrophages[23], the serum MCP-1 level was remarkably increased during infection-induced liver failure progression (Supplementary Figure 2B).
Figure 2.
Dynamic alteration of macrophage subsets during viral fulminant hepatitis progression. A: Time-course H&E staining on liver sections of mice after murine hepatitis virus strain 3 infection. Arrowheads indicated hepatocyte cytoplasmic destruction, Circles pointed to necrosis; B: ALT and AST levels from serum of mice post-viral infection; C: Flow cytometry of KCs (CD45+ F4/80high), MoMFs (CD45+ Ly6C+ F4/80int) of cells (left), and their percentage in the liver at various time point after viral infection; D: Flow cytometry of KCs (left) and frequency of M1 (iNOS+), M2 (CD206+) macrophages in KCs (right); E: Representative image of MoMFs (left) and frequency of Ly6Chigh and Ly6Clow MoMFs at different time point after viral infection. Data are presented as mean ± SD (n = 5). These experiments were repeated at least three times. KCs: Kuffer cells; MoMFs: Monocyte derived macrophages.
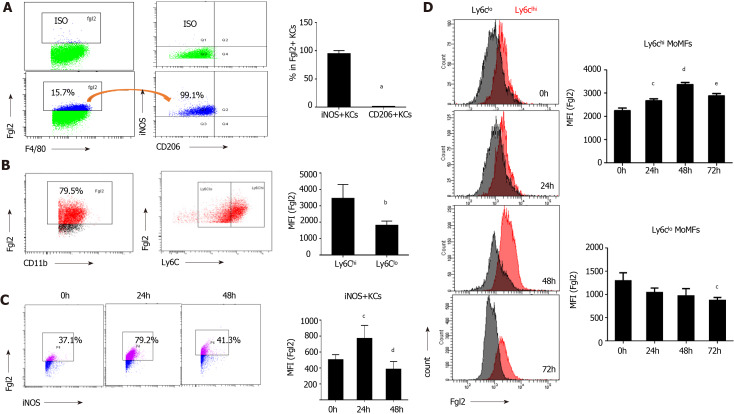
Potent induction of FGL2 is associated with the proinflammatory phenotype of both KCs and MoMFs in response to viral infection
As shown in Figure 3A, over 90% of FGL2-positive KCs were found in iNOS+ macrophages based on flow cytometry. Moreover, Ly6Chigh MoMFs constituted the major population of FGL2-positive MoMFs, and the expression level of FGL2 was remarkably higher in Ly6Chigh MoMFs than in Ly6Clow MoMFs (Figure 3B). After viral infection, sequential induction of FGL2 expression was observed in both polarized M1 KCs and Ly6Chigh MoMFs during disease progression, although a slight decrease in the expression level was observed when the KC and MoMF populations were somewhat exhausted at 48 and 72 h post viral infection, respectively (Figure 3C and D). However, FGL2 expression was maintained at low levels in Ly6Clow populations (Figure 3D). Together, these data suggested that FGL2 is markedly induced in proinflammatory macrophages upon viral infection.
Figure 3.
Fibrinogen-like protein 2 expression was robustly inducted upon proinflammatory macrophage activation. A: Flow cytometry of fibrinogen-like protein 2 (Fgl2) +KCs expressing iNOS and CD206 under homeostatic conditions (left), and frequency of iNOS+ and CD206+ cells subsets (right); B: Representative image of Fgl2+MoMFs expressing Ly6C (left), and relative expression level of Fgl2 in Ly6ChiMoMFs and Ly6Clo MoMFs under physiological conditions (right); C: Time course-presentation of Fgl2 expression in M1 polarized KCs (left), and expression level of Fgl2 in Ly6ChiMoMFs and Ly6Clow MoMFs, respectively (right); D: Cell counts of Fgl2+ MoMFs (left), and relative level of Fgl2 expression on both MoMFs subset (right) following viral infection. Data are presented as mean ± SD (n = 5). These experiments were repeated at least three times. MFI: Mean fluorescence intensity.
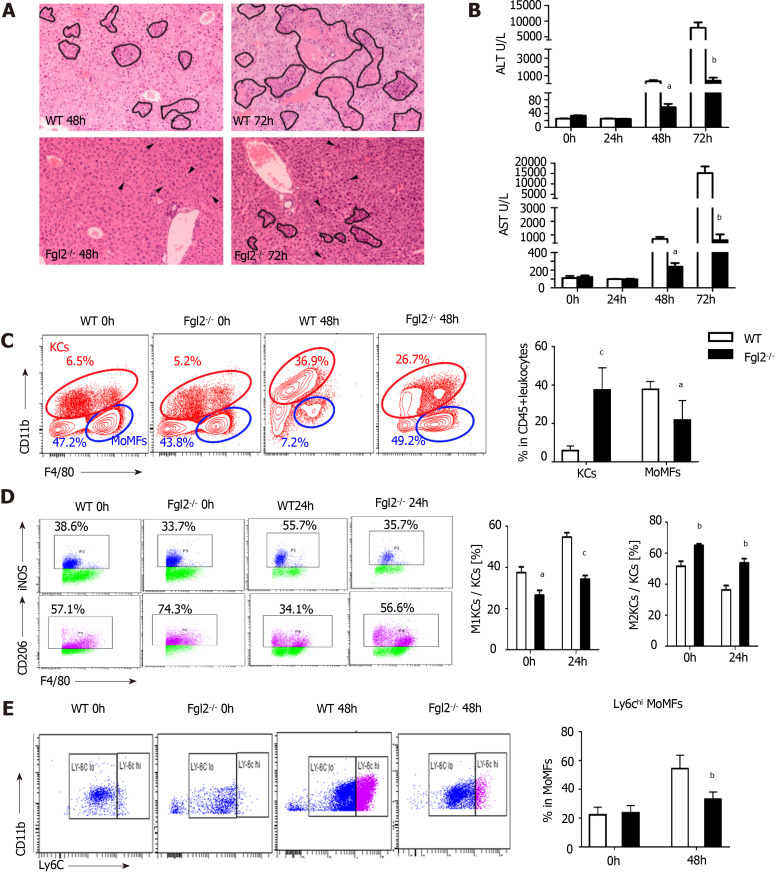
FGL2 deficiency prevents proinflammatory macrophage activation after MHV-3 infection
In Fgl2-/- mice (Supplementary Figure 3A), liver damage following MHV-3 infection was significantly attenuated, as revealed by narrowed necrosis foci and lower alanine transaminase (ALT) and aspartate transaminase (AST) levels compared with those in WT mice (Figure 4A and B). In addition, viral titers were reduced in Flg2-/- mice compared with those in their WT counterparts (Supplementary Figure 3B). Consistent with the pathology results, monocyte infiltration was significantly reduced in Flg2-/- mice, whereas a large number of KCs was maintained during VFH progression (Figure 4C). To determine whether Fgl2 was required for proinflammatory macrophage activation, we investigated the polarization of KCs at the early stage of VFH when KC populations were reserved. We found a decrease in the M1 KC subset and an increase in the M2 KC numbers in FGL2-deficient mice before and after MHV-3 infection (Figure 4D), suggesting that FGL2 depletion impaired inflammatory macrophage activation and favored the M2 phenotype. As expected, a small Ly6Chigh subset was also observed in the MoMF population in FGL2-deficient mice after viral infection, implying that Fgl2 loss predisposed MoMFs to the Ly6Clow phenotype (Figure 4E). This interpretation was further supported by the reduced levels of TNF-α and IL-6 and the increased levels of TGF-β on KCs from Fgl2-/- mice after viral infection compared with those in WT mice (Supplementary Figure 3C).
Figure 4.
Fibrinogen-like protein 2 promotes pro-inflammatory macrophage polarization following murine hepatitis virus strain 3 infection. A: H&E staining on liver sections from wild type (WT) and fibrinogen-like protein 2 (Fgl2-/-) mice at 48 and 72 h post viral infection. Circled field represented bulk necrosis. Arrows point to necrotic cells; B: Serum alanine transaminase and aspartate transaminase levels at 0, 24, 48, and 72 h post viral infection in WT and Fgl2-/- mice; C: Flow cytometry of KCs and MoMFs in hepatic CD45+ leukocytes at steady condition and 48 h following viral fulminant hepatitis in WT and Fgl2-/- mice (left); and respective frequency at 48 h post infection; D: Frequency of polarized M1 (iNOS+), M2 (CD206+) KCs at 0 and 24 h post murine hepatitis virus strain 3 (MHV-3) infection; E: Frequency of Ly6Chi and Ly6Clow MoMFs at 0 and 48 h post MHV-3 infection. Data are presented as mean ± SD (n = 5). These experiments were repeated at least three times.
FGL2 induces KC polarization for initiation of inflammatory events after viral infection
Macrophage depletion by clodronate liposomes significantly relieved liver injury, as shown by decreased necrosis foci and ALT/AST levels, in both WT and FGL2-deficient mice (Figure 5A and B). Notably, further reduction in liver ALT/AST levels and fewer necrotic hepatocytes were observed in FGL2-deficient mice after viral infection, suggesting the requirement of FGL2 for macrophage polarization and disease progression. To determine whether FGL2 regulates macrophage polarization directly, BMDMs from WT and Fgl2-/- mice were transferred into WT recipient mice with self-BMDMs depleted in advance in the VFH model. Interestingly, Fgl2-/- BMDMs exhibited reduced numbers of inflammatory polarized macrophages and increased numbers of anti-inflammatory macrophages (Figure 5D and E). Moreover, recipient mice adoptively transferred with Fgl2-/- BMDMs exhibited reduced liver injury, as revealed by histological analysis (Figure 5A) and ALT/AST levels (Figure 5C), when compared with that in recipients transferred with WT BMDMs.
Figure 5.
Depletion of macrophages in fibrinogen-like protein 2 synergically attenuated liver damage after viral infection. A: H&E staining on liver sections from mice administered with either clodronate liposomes (CL) (b and d on the right) or PBS-liposomes (PBS) (a and c on the left) at 48 h post murine hepatitis virus strain 3 (MHV-3) infection; e-f, liver sections from bone marrow-derived macrophages (BMDMs) adoptive transferred mice at 48 h post MHV-3 infection (e: WT BMDM donor; f: Fibrinogen-like protein 2 (Fgl2-/-) BMDM donor); B: Serum ALT and AST levels from clodronate liposomes-treated WT and Fgl2-/- mice at 48 h post MHV-3 infection; C: Serum AST and ALT levels from WT and Fgl2-/- BMDM chimeric mice; D: representative F4/80+ iNOS+ and F4/80+ CD206+ donor macrophages from WT and Fgl2-/- BMDM chimeric mice at 48 h post infection; E: statistical analysis of iNOS+ and CD206+ donor macrophages in chimeric mice at 48 h post infection; F: H&E-stained liver section from mice which were treated with Cenicriviroc (CVC) in advance and subjected to MHV-3 infection for 48 h. Arrows represented necrotic cells, circles represent areas of hepatocyte necrosis. Image magnificence: 200×; G: aminotransferase levels of CVC treated viral fulminant hepatitis mice and its control. Data were presented as mean ± SD (n = 5). These experiments were repeated at least three times. BMDM: Bone marrow derived macrophages.
As depicted in Supplementary Figure 3D and E, smaller numbers of MPOhigh myeloid cells, which are considered to be neutrophils, were observed in liver sections from mice with macrophage depletion or in FGL2-deficient mice compared with those from untreated WT mice following MHV-3 infection. However, no significant difference in neutrophil abundance was observed between WT and Fgl2-/- mice with macrophage depletion.
Because MoMFs formed the largest component of myeloid cells during the acute stage and CCR2 is mainly expressed in proinflammatory monocytes (Ly6Chigh)[24], we speculated that MoMF-derived inflammation is a major source of hepatic inflammation. Indeed, treatment with the CCR2 inhibitor cenicriviroc significantly reduced the virus-induced liver damage (Figure 5F and G). Collectively, these data suggest that FGL2 directly regulates proinflammatory macrophage polarization and that infiltrated MoMFs are the major source of hepatic inflammation during acute liver injury.
FGL2 deficiency attenuates macrophage M1 polarization in vitro
To further determine whether FGL2 regulates macrophage polarization directly in vitro, we examined its regulatory effect on BMDMs and PEMs by LPS stimulation or MHV-3 infection. As expected, in WT BMDMs, robust production of cytokines and chemokines, such as IL-1β, TNF-α, IL-6, IL-12, and MCP-1, and reactive species, such as the NO-derived nitrite product (NO2-), were observed in response to either LPS treatment or MHV-3 infection. In contrast, the levels of such products were markedly reduced in BMDMs from Fgl2-/- mice under the same condition (Figure 6A). Consistently, the transcription levels of M1 indicators, such as NOS2, TNF-α, IL-6, IL-1β, IL-12, and Marco, were significantly reduced in BMDMs of Fgl2-/- mice compared with those in BMDMs from WT mice in response to either LPS stimulation or MHV-3 infection (Figure 6B and C). In contrast, both IL-10 production and the transcriptional levels of M2 markers were significantly higher in Fgl2-/- BMDMs than in WT BMDMs in response to IL-4 treatment (Figure 6D and E). Similar results were also obtained in Fgl2-/- PEMs in response to LPS treatment (Supplementary Figure 4). Taken together, these data suggest that FGL2 deficiency impairs the proinflammatory polarization of macrophages and promotes an alternative activated phenotype in response to IL-4 stimulation.
Figure 6.
Fibrinogen-like protein 2 promoted bone marrow-derived macrophages M1 polarization in vitro. A: Supernatant concentration of NO2-, IL-1β, TNF-α, IL -6, IL-12 and MCP-1 released by bone marrow-derived macrophages (BMDMs) after LPS (100 ng/mL) treatment or murine hepatitis virus strain 3 (MHV-3) infection for 24 h; B: Relative transcriptional level of M1 markers (NOS2, IL-6, IL-12, IL-1β, TNF-α and marco) of WT and fibrinogen-like protein 2 (Fgl2-/-) BMDMs after the stimulation of LPS for 6 hours; C: Relative transcriptional level of M1 markers of WT and Fgl2-/- BMDMs after MHV-3 infection for 8 hours; D: Supernatant IL-10 concentration of BMDMs culture after the IL-4 stimulation (20 ng/mL) for 72 h; E: mRNA expression of M2 markers (Fizz1, ARG1, YM-1, MRC1, IL-10 and TGF-β) of WT and Fgl2 -/- BMDMs after of IL-4 treatment for 72 h. All data are presented as mean ± SD (n > 3). These experiments were repeated at least three times.
FGL2 deficiency reduces the antigen presentation and phagocytosis of macrophages
A critical function of hepatic macrophages during homeostasis maintenance is to engulf pathogens, endotoxins, and debris of dead cells via a process called phagocytosis, followed by antigen presentation by major histocompatibility complex (MHC) II for T cells. We therefore cultured E. coli labeled with red fluorescence together with peritoneal macrophages to examine their phagocytic capabilities. The number of engulfed bacteria was notably smaller in macrophages from FGL2-deficient mice than in macrophages from WT mice, although more macrophages were detected in Fgl2-/- mice (Figure 7A and B). Furthermore, surface expression of MHC II was more severely impaired in FGL2-deficient macrophages than in WT macrophages under normal conditions or after viral stimulation (Figure 7C), whereas expression of CD80, CD86, or MHC I was not altered (data not shown). These data suggest that FGL2 deficiency attenuates both the phagocytic capacity and antigen presentation potential of macrophages.
Figure 7.
Fibrinogen-like protein 2 deficiency resulted in low expression of major histocompatibility complex II and impaired phagocytosis of macrophage. A: Absolute number of PEMs under the stimulation of 3% starch broth or murine hepatitis virus strain 3 (MHV-3) extracted from wild-type (WT) and fibrinogen-like protein 2 (Fgl2-/-) mice; B: MFI of FITC+ phagocyted E.Coli by PEMs from WT and Fgl2-/- mice at different time points; C: Expression of major histocompatibility complex II on WT and Fgl2-/- PEMs under the stimulation of 3% starch broth or MHV-3 infection. All data are presented as mean ± SD. These experiments were repeated at least three times. PEMs: Peritoneal exudate macrophages.
FGL2 modulates the inflammatory signaling cascade by activating NF-κB, IRF3, IRF7, and p38
To explore how FGL2 modulates inflammatory cascades, we examined the serial components of inflammatory signaling pathways in BMDMs by mimicking invading pathogen infections in vitro. Consistent with the results from MoMFs in the VFH model, FGL2 was robustly induced in BMDMs following LPS treatment and MHV-3 infection (Figure 8A). In addition, phosphorylation of IκBα, p65, IRF3, and IRF7, which are critical modulators of NF-κB activation and IFN expression, was remarkably impaired in Fgl2-/- BMDMs in response to LPS treatment and viral infection (Figure 8B, Supplementary Figure 5). However, the expression of adaptor proteins such as TRIF and MyD88 was not altered under the same conditions (Figure 8B). Among mitogen-activated protein kinases (MAPKs), the level of phosphorylated p38 was reduced, whereas the levels of activated (phosphorylated) JNK and ERK were not affected in Fgl2-/- BMDMs compared with those in WT BMDMs following LPS treatment and viral infection (Figure 8C, Supplementary Figure 5), suggesting that FGL2 induces a positive feedback proinflammatory loop through p38 activation. Phosphorylation of p65 at Ser276 by c-Raf1 enables the recruitment of acetyltransferase CREB-binding protein (CBP) and p300, which leads to the acetylation of p65 and thus an enhanced NF-κB transcription rate[16]. Interestingly, phosphorylation of c-Raf1 and p65 (Ser276) and acetylation of p65 were reduced in Fgl2-/- BMDMs compared with those in WT BMDMs following either LPS stimulation or viral infection (Figure 8D). Phosphorylation of TBK, a modulator downstream of SYK that is involved in C-type lectin receptor (CLR)/Toll-like receptor (TLR) signaling-mediated innate immunity[25], did not differ between WT and Fgl2-/- BMDMs following LPS and MHV-3 challenge (Figure 8E). Taken together, our data suggest that induction of FGL2 in macrophages regulates inflammatory signaling by modulating p38, IRF3, IRF7, and p65 phosphorylation.
Figure 8.
Fibrinogen-like protein 2 deficiency impaired inflammatory cascade in response to lipopolysaccharide treatment of viral infection. A: Immunoblot for fibrinogen-like protein 2 (Fgl2) and β-actin expression in wild-type (WT) BMDMs in response lipopolysaccharide (LPS) stimulation and murine hepatitis virus strain 3 (MHV-3) infection. B-E: Immunoblot for proteins panels from lysates of WT and Fgl2-/- BMDMs in response to LPS treatment and MHV-3 infection; B: MyD88, TRIF, IκBα, phosphorylated IκBα, phosphorylated p65 (S468) and IRF3, phosphorylated IRF3, and IRF7; C: p38, JNK, ERK and phosphorylated p38, JNK, ERK; D: Phosphorylated c-Raf, phosphorylated p65 (S276), acetyl p65 (lys310); E: BTK, and phosphorylated BTK (Y223), BTK (Tyr550). These experiments were repeated at least three times.
DISCUSSION
Fulminant hepatitis, the most severe form of acute viral hepatitis, is a type of ALF induced by viral infection. Mounting evidence suggests that leukocyte infiltration initiates inflammation and liver damage in chronic viral hepatitis and alcoholic and non-alcoholic hepatitis with acute liver injury. However, there is no ideal experimental model to completely mimic the specific clinical manifestations of acute viral hepatitis. Most studies to date have focused on acute hepatitis B, in which virus-specific cytotoxic T lymphocytes (CTLs) mediate liver damage subsequent to viremia[26,27]. Similarly, non-virus-specific CD8+ T cells with innate-like cytolytic activity promote liver damage in patients with acute hepatitis A[28]. The innate immune response is involved in the early stage of viral clearance. Among innate immune cells, natural killer (NK) cells are preferentially studied because of their potent capacity to direct viral clearance as well as liver injury. Evidence from experimental VFH models and patients with acute viral hepatitis has revealed the detrimental contribution of NK cells to disease outcome[29,30]. Macrophages are the largest population of non-parenchymal immune cells in the liver, which proliferate and are activated in patients with acute hepatitis E virus infection and associated ALF, although their function is somewhat impaired[31]. The development of VFH is associated with hepatic macrophage replenishment by infiltrating macrophages, suggesting that macrophage infiltration is a fundamental event in VFH progression[32]. However, the pathological involvement of innate immunity in the liver damage occurring during acute viral hepatitis is not fully understood.
Experimental VFH established by either the coronavirus MHV-3 or infection with other hepatitis-causing viruses induces acute liver injury followed by ALF in 3-5 d in different strains of mice[12,14]. This suggests that adaptive immunity may not be the central event in disease progression, because of the rapid mortality despite infiltration of T cells to the liver[33]. In addition to monocytes, dendritic cells and neutrophils are also recruited to the liver during the progression of VFH, although the characteristics of the infiltrating leukocytes and their respective contributions to inflammation are unclear[12]. Neutrophils are a small subset of leukocytes that are required for monocyte infiltration during liver injury in mouse models of non-alcoholic steatohepatitis, acetaminophen overdose, and hepatitis virus infection[9,12,24]. In this study, we found that MoMFs dominated the population of infiltrating leukocytes, with a proinflammatory Ly6Chigh MoMF subset comprising the majority of the population, suggesting that targeting the transition from Ly6Chigh MoMFs to Ly6Clow MoMFs may be a promising strategy for treating VFH. Notably, the continued decrease in KC numbers during VFH suggests that necrosis occurred not only in hepatocytes but also in non-parenchymal cells.
FGL2 deficiency has been shown to prevent fulminant hepatitis following MHV-3 infection, which otherwise causes ALF with 100% mortality[14]. Moreover, FGL2 expression is largely distributed in and robustly induced by macrophages, in spite of moderate expression in dendritic cells and endothelial cells following viral infection[7]. The mechanism by which FGL2 contributes to VFH is generally considered to involve its procoagulant activity[14,34]. However, reciprocal activation between inflammation mediators or cytokines and tissue factor-mediated fibrin deposition and thrombin generation, the major contributor to inflammation-initiated coagulation, is largely dependent on IL-6[11,35]. It is therefore difficult to determine whether co-localization between fibrin deposition and FGL2 expression solely results from the prothrombinase activity of FGL2. In addition, inflammation may be induced by tissue factors secreted by local endothelial and immune cells[36]. Proinflammatory macrophage polarization is a process resulting from the Th1 response and pattern recognition receptor (PRR)-mediated signaling events initiated by PAMPs or DAMPs[37]. Unlike soluble FGL2 expressed by regulatory T cells and Th2-like immune cells with immunosuppressive activity, membrane FGL2 can amplify the classical inflammatory cascades and may act as a co-receptor to cooperate with TLRs or other PPRs for signal transduction. Our results suggest that FGL2 deficiency may foster a default transition from proinflammatory macrophages to an alternative activated phenotype because more M2 macrophages and Ly6Clo MoMFs were observed under normal conditions. The inflammatory cytokines secreted by endothelial and dendritic cells in which FGL2 is expressed may also play a role; however, compared to macrophages, these cells are in relatively low abundance[38].
The innate immune responses triggered by viral infection are largely regulated by PPR-mediated signaling, which depends on cytosolic adaptors (e.g., TRIF and MyD88) and kinase-dependent factors[39]. Macrophage M1 polarization promotes the activation and translocation of NF-κB and the IRF3/IRF7 complex to initiate the transcription of Th1-associated gene targets[40]. p38 MAPKs are known to modulate PPR signaling by collaborating with NF-κB-mediated transcription[33]. CLRs are another type of PPR that modulate TLR-mediated signaling in the defense against invading pathogens[14]. In invertebrates, numerous molecules containing fibrinogen-related domains participate in immune response transcription and have been shown to act as PPRs in defense processes, such as agglutination, and to cooperate with CLRs to synergistically clear pathogens[41]. Thus, we questioned whether FGL2 regulated TLR signaling in a CTL-like manner. MyD88-dependent PPR signaling involves activation of members of the IRAK family, which in turn stimulates the E3 ligase activity of TRAF6, enabling activation of the downstream ubiquitin-dependent kinase TAK1[42]. Upon activation, TAK1 activates MAPK and its downstream kinase IKK, which in turn phosphorylates the NF-κB inhibitor IκBα, leading to ubiquitin-dependent IκBα degradation and subsequent NF-κB activation[42]. Thus, TRIF-dependent PPR signaling involves TRAF3 recruitment and activation of TBK1 and IKKɛ, which stimulate IRF3 phosphorylation and NF-κB activation, thereby leading to the transcriptional induction of type I IFNs and inflammatory cytokines[43]. Membrane proteins that regulate membrane PPR-mediated signaling function as co-receptors or factors involved in TLR assembly, internalization, or trafficking[44-47]. Accordingly, FGL2 may act as an assembly factor, could interact with PPRs for ligand-receptor binding, or even mediate the trafficking between the membrane and endosome, thereby facilitating inflammatory signaling transduction. Previous data showed that MHV-3-induced FGL2 expression in macrophages relies on p38 activation[48]. In this work, we found that FGL2 is needed for p38 phosphorylation, suggesting that FGL2 expression provides a positive feedback loop for proinflammatory aggregation in macrophages.
The exact role of resident macrophages and MoMFs in VFH is obscure. We speculate that resident macrophages may initiate liver inflammation during the early stage of viral infection because MoMFs exacerbate liver damage during the acute phase. Resident macrophages represent the first line of defense against viral infection. Once primed, resident macrophages produce chemotaxins and cytokines that recruit and activate the MoMFs, and our results suggest that FGL2 expression on macrophages prompts and enhances their inflammatory phenotype.
CONCLUSION
Our data revealed, for the first time, that Ly6Chigh MoMF infiltration is a critical event for hepatic inflammatory accumulation and subsequent liver damage during virus-induced hepatitis progression. FGL2 expression is required for maintaining the proinflammatory Ly6Chigh MoMF phenotype by mediating IRF3, p65, and p38 phosphorylation, thereby forming a positive feedback loop of inflammatory accumulation in the liver.
ARTICLE HIGHLIGHTS
Research background
Viral fulminant hepatitis (VFH) is a devastating syndrome that pathologically caused by excessive activation of both innate and adaptive immunity. However, the extent of contribution of innate immunity in VFH is not well defined. Macrophage polarization have been implicated in host defense and the pathogenesis of various hepatic diseases. Fibrinogen-like protein 2 (FGL2) can be induced robustly and exclusively in macrophages in response to cytokines or viral infection. Exploring their roles in VFH can greatly improve our understanding of the disease and thus seek the therapeutic approaches.
Research motivation
Hepatic macrophages are attractive therapeutic targets because their functions can be inhibited or augmented to alter disease outcomes. A better understanding of their biological properties and immunologic function in liver homeostasis and pathology may pave the way for new diagnostic and therapeutic approaches for liver failure or other liver diseases.
Research objectives
To evaluate the role of Fgl2 in the reconstitution of hepatic macrophages during VFH progression by the VFH mouse model.
Research methods
Liver sections of liver failure patients and controls were immuno-stained for macrophages examination. Murine hepatitis virus strain 3 (MHV-3) was used to induce VFH experimental model in wild type and Fgl2-/- mice. Adoptive transfer or depletion of macrophages were employed to assess liver damage and hepatic macrophages alteration. Signal cascade induced by LPS or MHV-3 were detected in macrophages.
Research results
Infiltrated MoMFs is a major source of hepatic inflammation during VFH progression. Fgl2 expression on macrophages prompts and enhances the inflammatory phenotype of hepatic macrophages, which breaks the previous understanding that the mechanism of Fgl2 during VFH is generally considered to be procoagulant activity.
Research conclusions
We revealed for the first time that pro-inflammatory monocyte-derived macrophages (MoMFs) infiltration is critical event for hepatic inflammatory accumulation and subsequent liver damage during virus-induced hepatitis progression and Fgl2 is required for maintaining the pro-inflammatory hepatic macrophages phenotype.
Research perspectives
Macrophages are ‘keystones’ of liver architecture in both homeostasis and disease. The development of potential therapies highly depends on a fundamental knowledge about the mechanisms that trigger the polarization and control the fate of hepatic macrophages. We believe that a better understanding of the specific mechanisms underlying macrophages participation in diseases will definitely result in the increased efficacy of these therapies.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board at Tongji Hospital, Wuhan Province.
Institutional animal care and use committee statement: All animal experiments were approved by institutional Animal Care and Use Committee of Tongji Hospital (permit number: TJ-A20171008), and conducted in accordance with state guidelines from the Ministry of Science and Technology of China.
Conflict-of-interest statement: All authors declare no conflict of interest in the study.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Asian Pacific Association of Gastroenterology.
Peer-review started: August 18, 2021
First decision: December 4, 2021
Article in press: January 8, 2022
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Makishima M S-Editor: Wang LL L-Editor: A P-Editor: Guo X
Contributor Information
Fang Xiao, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China; Department of Infectious Disease, The First Affiliated Hospital of Guangxi Medical University, Nanning 530000, Guangxi Province, China.
Hong-Wu Wang, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Jun-Jian Hu, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Ran Tao, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Xin-Xin Weng, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Peng Wang, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Di Wu, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Xiao-Jing Wang, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Wei-Ming Yan, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Dong Xi, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Xiao-Ping Luo, Department of Pediatrics, Tongji Hospital, Wuhan 430030, Hubei Province, China.
Xiao-Yang Wan, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China.
Qin Ning, Department of Infectious Disease, Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei Province, China. qning@vip.sina.com.
Data sharing statement
No additional data are available.
References
- 1.Manka P, Verheyen J, Gerken G, Canbay A. Liver Failure due to Acute Viral Hepatitis (A-E) Visc Med. 2016;32:80–85. doi: 10.1159/000444915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–6172. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Ning Q. Immune mediated liver failure. EXCLI J. 2014;13:1131–1144. [PMC free article] [PubMed] [Google Scholar]
- 4.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondo Y, Kobayashi K, Asabe S, Shiina M, Niitsuma H, Ueno Y, Kobayashi T, Shimosegawa T. Vigorous response of cytotoxic T lymphocytes associated with systemic activation of CD8 T lymphocytes in fulminant hepatitis B. Liver Int. 2004;24:561–567. doi: 10.1111/j.1478-3231.2004.0982.x. [DOI] [PubMed] [Google Scholar]
- 6.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43:S54–S62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 7.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol. 2016;13:316–327. doi: 10.1038/cmi.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. doi: 10.1016/j.jhep.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 9.Bashir S, Sharma Y, Elahi A, Khan F. Macrophage polarization: the link between inflammation and related diseases. Inflamm Res. 2016;65:1–11. doi: 10.1007/s00011-015-0874-1. [DOI] [PubMed] [Google Scholar]
- 10.Zigmond E, Samia-Grinberg S, Pasmanik-Chor M, Brazowski E, Shibolet O, Halpern Z, Varol C. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J Immunol. 2014;193:344–353. doi: 10.4049/jimmunol.1400574. [DOI] [PubMed] [Google Scholar]
- 11.Ju C, Reilly TP, Bourdi M, Radonovich MF, Brady JN, George JW, Pohl LR. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem Res Toxicol. 2002;15:1504–1513. doi: 10.1021/tx0255976. [DOI] [PubMed] [Google Scholar]
- 12.Tang Y, Li H, Li J, Liu Y, Li Y, Zhou J, Lu X, Zhao W, Hou J, Wang XY, Chen Z, Zuo D. Macrophage scavenger receptor 1 contributes to pathogenesis of fulminant hepatitis via neutrophil-mediated complement activation. J Hepatol. 2018;68:733–743. doi: 10.1016/j.jhep.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Mendicino M, Ning Q, Ghanekar A, He W, McGilvray I, Shalev I, Pivato D, Clark DA, Phillips MJ, Levy GA. Cytokine-induced hepatic apoptosis is dependent on FGL2/fibroleukin: the role of Sp1/Sp3 and STAT1/PU.1 composite cis elements. J Immunol. 2006;176:7028–7038. doi: 10.4049/jimmunol.176.11.7028. [DOI] [PubMed] [Google Scholar]
- 14.Marsden PA, Ning Q, Fung LS, Luo X, Chen Y, Mendicino M, Ghanekar A, Scott JA, Miller T, Chan CW, Chan MW, He W, Gorczynski RM, Grant DR, Clark DA, Phillips MJ, Levy GA. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J Clin Invest. 2003;112:58–66. doi: 10.1172/JCI18114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu GL, Chen J, Yang F, Li GQ, Zheng LX, Wu YZ. C5a/C5aR pathway is essential for the pathogenesis of murine viral fulminant hepatitis by way of potentiating Fgl2/fibroleukin expression. Hepatology. 2014;60:114–124. doi: 10.1002/hep.27114. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Tan Y, Zhang J, Zou L, Deng G, Xu X, Wang F, Ma Z, Zhao T, Liu Y, Li Y, Zhu B, Guo B. C5aR, TNF-α, and FGL2 contribute to coagulation and complement activation in virus-induced fulminant hepatitis. J Hepatol. 2015;62:354–362. doi: 10.1016/j.jhep.2014.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Sarin SK, Choudhury A. Acute-on-chronic liver failure: terminology, mechanisms and management. Nat Rev Gastroenterol Hepatol. 2016;13:131–149. doi: 10.1038/nrgastro.2015.219. [DOI] [PubMed] [Google Scholar]
- 18.Sarin SK, Kedarisetty CK, Abbas Z, Amarapurkar D, Bihari C, Chan AC, Chawla YK, Dokmeci AK, Garg H, Ghazinyan H, Hamid S, Kim DJ, Komolmit P, Lata S, Lee GH, Lesmana LA, Mahtab M, Maiwall R, Moreau R, Ning Q, Pamecha V, Payawal DA, Rastogi A, Rahman S, Rela M, Saraya A, Samuel D, Saraswat V, Shah S, Shiha G, Sharma BC, Sharma MK, Sharma K, Butt AS, Tan SS, Vashishtha C, Wani ZA, Yuen MF, Yokosuka O APASL ACLF Working Party. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol Int. 2014;8:453–471. doi: 10.1007/s12072-014-9580-2. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto K, Minagawa H, Yanagi Y. Caspase-dependent apoptosis in fulminant hepatic failure induced by herpes simplex virus in mice. J Hepatol. 2003;39:773–778. doi: 10.1016/s0168-8278(03)00385-4. [DOI] [PubMed] [Google Scholar]
- 20.Heymann F, Hammerich L, Storch D, Bartneck M, Huss S, Rüsseler V, Gassler N, Lira SA, Luedde T, Trautwein C, Tacke F. Hepatic macrophage migration and differentiation critical for liver fibrosis is mediated by the chemokine receptor C-C motif chemokine receptor 8 in mice. Hepatology. 2012;55:898–909. doi: 10.1002/hep.24764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, Heymann F, Kalthoff S, Lefebvre E, Eulberg D, Luedde T, Marx G, Strassburg CP, Roskams T, Trautwein C, Tacke F. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology. 2016;64:1667–1682. doi: 10.1002/hep.28682. [DOI] [PubMed] [Google Scholar]
- 23.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel B, Bansal SS, Ismahil MA, Hamid T, Rokosh G, Mack M, Prabhu SD. CCR2+ Monocyte-Derived Infiltrating Macrophages Are Required for Adverse Cardiac Remodeling During Pressure Overload. JACC Basic Transl Sci. 2018;3:230–244. doi: 10.1016/j.jacbts.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber ANR, Bittner Z, Liu X, Dang TM, Radsak MP, Brunner C. Bruton's Tyrosine Kinase: An Emerging Key Player in Innate Immunity. Front Immunol. 2017;8:1454. doi: 10.3389/fimmu.2017.01454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C- monocytes. Nat Immunol. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thimme R, Wieland S, Steiger C, Ghrayeb J, Reimann KA, Purcell RH, Chisari FV. CD8 (+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77:68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim J, Chang DY, Lee HW, Lee H, Kim JH, Sung PS, Kim KH, Hong SH, Kang W, Lee J, Shin SY, Yu HT, You S, Choi YS, Oh I, Lee DH, Jung MK, Suh KS, Hwang S, Kim W, Park SH, Kim HJ, Shin EC. Innate-like Cytotoxic Function of Bystander-Activated CD8+ T Cells Is Associated with Liver Injury in Acute Hepatitis A. Immunity. 2018;48:161–173.e5. doi: 10.1016/j.immuni.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 29.Manickam C, Rajakumar P, Wachtman L, Kramer JA, Martinot AJ, Varner V, Giavedoni LD, Reeves RK. Acute Liver Damage Associated with Innate Immune Activation in a Small Nonhuman Primate Model of Hepacivirus Infection. J Virol. 2016;90:9153–9162. doi: 10.1128/JVI.01051-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Y, Chen T, Han M, Wang H, Yan W, Song G, Wu Z, Wang X, Zhu C, Luo X, Ning Q. Increased killing of liver NK cells by Fas/Fas ligand and NKG2D/NKG2D ligand contributes to hepatocyte necrosis in virus-induced liver failure. J Immunol. 2010;184:466–475. doi: 10.4049/jimmunol.0900687. [DOI] [PubMed] [Google Scholar]
- 31.Sehgal R, Patra S, David P, Vyas A, Khanam A, Hissar S, Gupta E, Kumar G, Kottilil S, Maiwall R, Sarin SK, Trehanpati N. Impaired monocyte-macrophage functions and defective Toll-like receptor signaling in hepatitis E virus-infected pregnant women with acute liver failure. Hepatology. 2015;62:1683–1696. doi: 10.1002/hep.28143. [DOI] [PubMed] [Google Scholar]
- 32.Borst K, Frenz T, Spanier J, Tegtmeyer PK, Chhatbar C, Skerra J, Ghita L, Namineni S, Lienenklaus S, Köster M, Heikenwaelder M, Sutter G, Kalinke U. Type I interferon receptor signaling delays Kupffer cell replenishment during acute fulminant viral hepatitis. J Hepatol. 2018;68:682–690. doi: 10.1016/j.jhep.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Wu S, Guo G, Fei L, Guo S, Yang C, Fu X, Wu Y. Programmed death (PD)-1-deficient mice are extremely sensitive to murine hepatitis virus strain-3 (MHV-3) infection. PLoS Pathog. 2011;7:e1001347. doi: 10.1371/journal.ppat.1001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding JW, Ning Q, Liu MF, Lai A, Leibowitz J, Peltekian KM, Cole EH, Fung LS, Holloway C, Marsden PA, Yeger H, Phillips MJ, Levy GA. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J Virol. 1997;71:9223–9230. doi: 10.1128/jvi.71.12.9223-9230.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Poll T, Levi M, Hack CE, ten Cate H, van Deventer SJ, Eerenberg AJ, de Groot ER, Jansen J, Gallati H, Büller HR. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253–1259. doi: 10.1084/jem.179.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauch U, Bonderman D, Bohrmann B, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Transfer of tissue factor from leukocytes to platelets is mediated by CD15 and tissue factor. Blood. 2000;96:170–175. [PubMed] [Google Scholar]
- 37.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 38.Yang C, Chen Y, Guo G, Li H, Cao D, Xu H, Guo S, Fei L, Yan W, Ning Q, Zheng L, Wu Y. Expression of B and T lymphocyte attenuator (BTLA) in macrophages contributes to the fulminant hepatitis caused by murine hepatitis virus strain-3. Gut. 2013;62:1204–1213. doi: 10.1136/gutjnl-2012-302239. [DOI] [PubMed] [Google Scholar]
- 39.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 40.Günthner R, Anders HJ. Interferon-regulatory factors determine macrophage phenotype polarization. Mediators Inflamm. 2013;2013:731023. doi: 10.1155/2013/731023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hanington PC, Zhang SM. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J Innate Immun. 2011;3:17–27. doi: 10.1159/000321882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- 43.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2 doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 45.He Z, Riva M, Björk P, Swärd K, Mörgelin M, Leanderson T, Ivars F. CD14 Is a Co-Receptor for TLR4 in the S100A9-Induced Pro-Inflammatory Response in Monocytes. PLoS One. 2016;11:e0156377. doi: 10.1371/journal.pone.0156377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brinkmann MM, Spooner E, Hoebe K, Beutler B, Ploegh HL, Kim YM. The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J Cell Biol. 2007;177:265–275. doi: 10.1083/jcb.200612056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tatematsu M, Funami K, Ishii N, Seya T, Obuse C, Matsumoto M. LRRC59 Regulates Trafficking of Nucleic Acid-Sensing TLRs from the Endoplasmic Reticulum via Association with UNC93B1. J Immunol. 2015;195:4933–4942. doi: 10.4049/jimmunol.1501305. [DOI] [PubMed] [Google Scholar]
- 48.McGilvray ID, Lu Z, Wei AC, Dackiw AP, Marshall JC, Kapus A, Levy G, Rotstein OD. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J Biol Chem. 1998;273:32222–32229. doi: 10.1074/jbc.273.48.32222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.