Abstract
Naringenin is one of the most important and abundant known flavonoids found in grapefruit and other citrus fruits. This experimental study aimed to assess the clinical effects and immune responses of naringenin in the animal model of rheumatoid arthritis (RA) according to various reports on its anti-inflammatory effects and modulation of the immune system. To this end, 40 Wistar rats in the weight range of 160-180g were randomly assigned to four groups (n=10) including healthy, control, naringenin, and methotrexate orally treated groups. To induce RA disease, a compound of 200 μl of Freund's adjuvant and collagen type II was injected subcutaneously into the rear footpads of rats. The severity of RA clinical signs was assessed based on a standard scoring method. The treatment lasted for three weeks (days7-28 after induction). The obtained data pointed out that the levels of C-reactive protein (CRP), myeloperoxidase, nitric oxide, IL-17, and IFN-γ cytokines significantly increased in the RA rats, while the level of their serum antioxidants significantly reduced, compared to the healthy rats. The inflammation of the paws and the level of CRP decreased similarly in both methotrexate and naringenin-treated groups. In the naringenin-treated group, a further decrease was detected in serum myeloperoxidase, nitric oxide, and the total antioxidant capacity occurred, as compared to the methotrexate-treated rats. Nonetheless, IL-17 and IFN-γ cytokines levels were further decreased in the methotrexate-treated group. Accordingly, it can be concluded that naringenin can be effectively used for the reduction of inflammatory effects and control of RA disease.
Keywords: Rheumatoid arthritis, Naringenin, Methotrexate, Wistar rats
1. Introduction
Rheumatoid arthritis (RA) is a systemic, chronic, and progressive disease ( 1 ) with a prevalence of 0.5%-1% in dogs and human population ( 2 ). The disease is characterized by the formation and deposition of immune complexes in the synovial tissue and other organs. It leads to inflammation, synovial thickening, pain, stiffness, swelling, deformity, displacement, reduced joint mobility, and development of immune-mediated inflammatory disease ( 3 , 4 ).
Dogs with RA also show lethargy, anorexia, and fever ( 5 ). Although the main cause of this disease is unknown, it is regarded as an autoimmune disease due to the presence of autoantibodies against citrulline proteins and other joint components ( 6 ). It has been demonstrated that the progression of the disease to areas outside the joints can cause complications in coronary arteries and cardiovascular disorders which are among the leading causes of death in patients with RA ( 7 ).
In the past, CD4+T lymphocytes appeared to play a critical role in Th-1 conversion and secretion of inflammatory cytokines in the development of RA; nonetheless, therapeutic measures against it do not reduce the severity of the disease. Since 2005, the prominent role of Th-17 (IL-17) has been considered a potent mediator in RA induction. The Th-17-producing cells express the transcription factor RORγt. These cells are differentiated in vitro by transforming growth factor-β (TGF-β) and interleukin 6 (IL-6) and proliferated in the presence of Interleukin-(IL)-23, IL-1, and tumor necrosis factor α (TNFα). Due to the regulatory role of Th-1 and Th-17, the lack of cytokines secreted by Th-1 intensifies the differentiation of Th-17.
Due to its pleiotropic role in various cells in synovial tissue, IL-17 synergistically increases the production of TNF-α, IL-1, and IL-6 by macrophages and fibroblasts ( 8 ). Although various drugs are used to treat RA, including glucocorticoids, nonsteroidal anti-inflammatory drugs, methotrexate, anti-B lymphocyte drugs, anti-cytokines, and CTLA-4 Ig, they are less desirable due to their adverse side effects or high cost, and many patients did not give positive responses. Therefore, ongoing research has aimed to find low-risk and low-cost drugs to add to patients' treatment regimens ( 9 , 10 ).
Naringenin (4, 5, 7-trihydroxyflavanone) is one of the most important and abundant known flavonoids found in grapefruit and other citrus fruits ( 11 , 12 ). Flavonoids are important plant pigments with numerous biological and pharmacological effects, including strong antioxidant properties ( 13 ). Therefore, naringenin can seemingly modulate acute and chronic inflammatory responses. There have also been reports of the effects of naringenin on controlling sepsis, acute hepatitis, fibrosis, colitis, autoimmune experimental encephalomyelitis, and even cancers ( 14 - 16 ).
Apart from anti-inflammatory effects, naringenin also exerts noticeable effects on lipoprotein metabolism which can be effective in controlling diabetes by decreasing insulin resistance and atherosclerosis ( 17 ). So far, no comprehensive research has been conducted on the possible effects of naringenin in patients with RA or its experimental model. Therefore, the current aimed to assess the effect of Naringenin on experimentally induced rheumatoid arthritis in Wistar rats.
2. Material and Methods
2.1. Analysis Method
All 40 male Wistar rats (160-180 g), used in this experimental study, were purchased from Razi Vaccine and Serum Research Institute. The rats were maintained at 25°C on a Light (L) phase–Dark (D) phase 12:12 cycle during the study period and had free access to water and food. The straw of cages was sterile and changed every two days. To adapt to environmental conditions, rats were kept in quarantine for three days. Thereafter, they were randomly assigned to four equal groups (n=10) as follows:
Healthy rat group: In this group, RA wasnot induced, and they did not receive any treatment except Phosphate buffered saline (PBS) (as a placebo) daily and orally in a volume of 0.5 ml.
Control rat group: In this group, RA was induced, and they received PBS daily and orally in a volume of 0.5 ml from the seventh day after the onset of the disease (observingswelling in the paw of therats).
Naringenin-treated rat group: In this group, RA was induced, and they receivednaringenin suspension in PBS (40 mg/kg, purchased from Chengdu Kanghui Biotechnology Co., Ltd) daily and orally in a volume of 0.5 ml from days 7-28 after induction.
Methotrexate-treated rat group: In this group, RA was induced and they received methotrexate suspension in PBS (1 mg/kg, purchased from Ebew company) daily and orally in a volume of 0.5 ml from days 7-28 after induction.
These doses of naringenin and methotrexate were based on previously performed studies ( 18 , 19 ). To induce RA, 200 μg of type II collagen (Sigma Aldrich) was dissolved in 100 μl of 0.05 M acetic acid solution, and 100 μl of Freund's adjuvant (Sigma Aldrich) was then added. Mixing was continued until the obtainment of a uniform emulsion. The emulsion (final volume of 200 μl) was injected subcutaneously into the rear footpads of rats. In the healthy group, the same volume of PBS was injected ( 20 ). From the 7th day, the clinical signs of induced RA (swelling, redness, and inflammation) were observed, and treatment protocols were initiated. In this study, the specific scoring method was used for each paw with a score range of 0-4 as follows:
Score 0: paw without swelling and redness
Score 1: paw with redness and slight swelling
Score 2: paw with moderate edema
Score 3: paw with prominent edema and limited use of the joint
Score 4: paw with severe edema and inability to use the joint.
The injected foot was removed from the scoring process. Therefore, the maximum score per rat could be 12 ( 9 ). The alterations in the paw diameter of each rat were recorded on days 7, 14, 21, and 28.On day 28, the animals underwent deep anesthesia and bled to get the serum for the following tests to be performed:
Myeloperoxidase (MPO) activity test: 10 μl of serum sample was mixed with80 μl of 0.75 mM hydrogen peroxide and 110 μl of the reaction solution containing 2.9 mmol of TMB in 14.5% dimethyl sulfoxide plus 150 mM of sodium phosphate buffer (pH = 4.5). The samples were kept at 37°C for 15 min; thereafter, 50 μl of sulfuric acid (2 M solution) was added to stop the reaction. Light absorbance was measured at 450 nm (reference: 620 nm). As a standard for positive control, 10 μl of HRP (Horseradish Peroxidase) (2.5 and 25 mU/ml) was used. Finally, MPO activity was reported based on the difference in absorbance, compared to the Horseradish peroxidase (HRP) standard curvein mIU/ml ( 21 ).
Nitric oxide measurement in serum samples:In brief, 100 μl of Griess solution (0.1% naphthyl ethylene diamide, 3% phosphoric acid, and 0.1% sulfanilamide) was mixed with 100 μlserum sample. The resulting mixture was kept in the dark at 25°C for 10 min. Finally, the light absorbance was read at 540 nm. The nitric oxide level was reported, compared to the standard curve (Griess method) ( 10 ).
Total antioxidant capacity (TAC): The TAC of serum samples was assessed by ferric reducing antioxidant power (FRAP) kitwhich measures the reducing potential of an antioxidant reaction with a ferric tripyridyltriazine (Fe3+-TPTZ) complex and produces a colored ferrous tripyridyltriazine (Fe2+-TPTZ)]. To this end, 20 μl of serum sample was added to 1 ml of working solution and vortexed. The optical absorption of the sample was read at 593 nm at zero time and four min later; thereafter, itwas compared to the control sample (Blank). Following that, the amount of light absorbancewas entered in the TAC formula to obtain its value. The numerical size of the TAC was calculated by the standard curve ( 22 ). Moreover, the sera levels of C-reactive protein (CRP) weremonitored by commercial C-reactive protein (CRP) measuring kits (BD, UK).
In order to evaluate the production of IL-17 and IFN-γ cytokines, on the 28th day after the induction of the disease, the spleens of rats were removed under sterile conditions. Each spleen was then crushed in 5 ml of RPMI1640 (Sigma) culture medium containing 10% FBS (Gibco) and passed through a wire mesh with a diameter of 20 μm. The resulting cell suspension was centrifuged at 2000 rpm for 10 min. Subsequently, 5 ml of RBS lysis buffer was added to the cell sediment, and after 5 min, it was centrifuged at 3000 rpm for 15 min by the addition of 10 ml of RPMI medium. Finally, the resulting cell sediment was suspended in RPMI1640 (10% FBS, 0.1 g/l penicillin, 0.1 g/l streptomycins, and 2.5 mg/l amphotericin B). Following cell counting by the Trypan Blue method, a suspension containing 10×105 cells/ml was prepared, and 100 μlof that was transferred into each well of the 96-well flat plate. Thereafter, collagen was added to each well at a final concentration of 50 μg/ml, and the cells were incubated for 72h at 37°C, 5% CO2 concentration, and 95% relative humidity ( 20 ). For each sample, three replications in the presence of collagen and three replications without the presence of collagen were considered. After 72 h, the plates were centrifuged, and the supernatantwas collected. The levels of IL-17 and IFN-γ cytokines were measured as usual by PeproTech ELISA kits.
Non-parametric data related to the arthritis index was analyzed using the Kruskal-Wallis test and subsequent Bonferroni Mann-Whitney U test. After the confirmation of the normal distribution of other data by the Kolmogorov-Smirnov test, these findings were evaluated using a one-way analysis of variance with the Tukey posthoc test. Data were reported as mean±standard deviationand analyzed in MedCalc statistical software (version 18.9.1).
3. Results
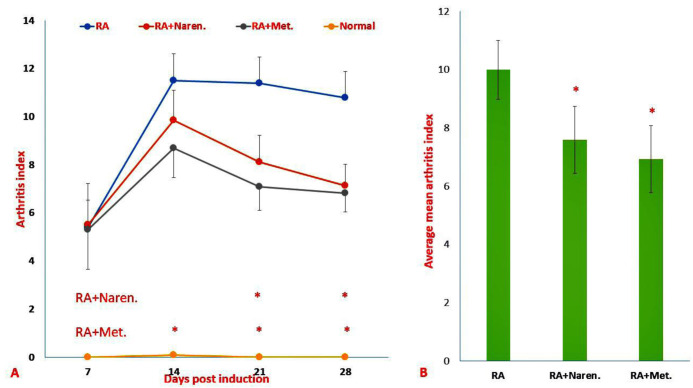
On the 7th day after disease induction,all the injected rats showed redness and swelling in the injected paw ( Figure 1). The analysis of the arthritis index (the sum of scores for the carpal and tarsal joints that had not been injected) demonstrated that the administration of methotrexate and naringenin reduced this index. This reduction occurred significantly from days 14 and 21 in the methotrexate treated and naringenin treated groups, respectively (P<0.05; Figure 1A). Nevertheless, according to Figure 1B, on the last day, the rats in the two mentioned groups did not significantly differ in the mean arthritis index (P=0.31).
Figure 1.
Assessment of clinical schemas in RA rats. A) Mean arthritis index, B) Average of mean arthritis. Findings were presented as mean ±S.D. (&p<0.05 versus healthy rats, *p<0.05 versus PBS-treated RA rats). (Naren. Naringenin-treated RA group; Met., methotrexate-treated RA group).
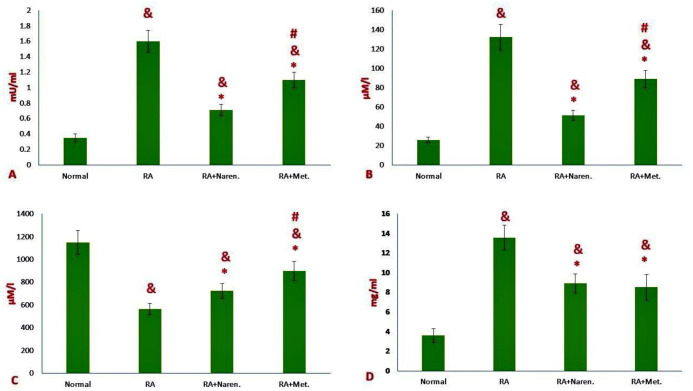
According to figure 2A, the level of myeloperoxidase enzyme in the serum of rats with rheumatoid arthritis displayed a significant increase, compared to that in healthy rats (P<0.05). Both methotrexate and naringenin reduced the levels of myeloperoxidase in the serum of treated rats, as compared to the control group (P<0.05). However, the rate of the reduction was higher in the naringenin-treated group, in comparison with that in the methotrexate-treated group (P<0.05; Figure 2A).
Figure 2.
Changes in the biochemical profiles of sera in RA rats. A) Myeloperoxidase activity (MPO), B) Nitric oxide (NO), C) total antioxidant capacity (TAC), and D) C-reactive protein (CRP). Data were reported as mean ±S. D. (&p<0.05 versus healthy rats, *p<0.05 versus PBS-treated RA rats; #p<0.05 versus methotrexate treated RA rats). (Naren. Naringenin-treated RA group; Met., methotrexate-treated RA group).
Serum nitric oxide levels also illustrated a significant increase in induced rats, compared to those in healthy ones (P<0.05; Figure 2B). Both Naringenin and Methotrexate treated groups displayed a decrease in serum nitric oxide (P<0.05). In the naringenin-treated group, the reduction rate of nitric oxide was higher than that in the methotrexate-treated group (P<0.05; Figure 2B).The results obtained from the sera of rats pointed to a significant reduction (an average of 61%) in the total serum antioxidant capacity, compared to that in healthy rats (P<0.05; Figure 2C). Although both treatment protocols were effective in this case, naringenin treatment resulted in more considerable improvement, as compared to methotrexate (P<0.05; Figure 2C).
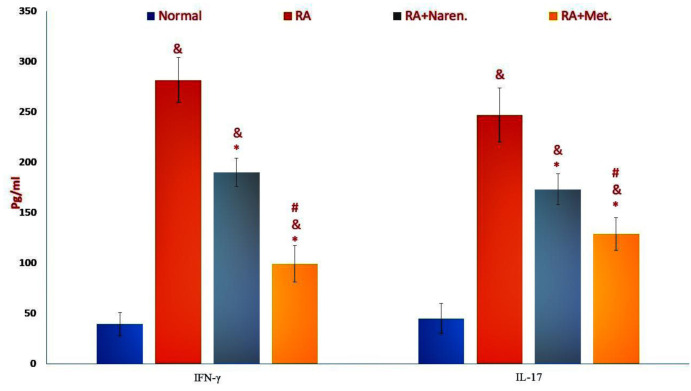
As expected, the induction of rheumatoid arthritis in rats could promote a significant increase in the CRP serum levels of affected rats ( Figure 3), indicating that therapeutic regimens were effective in the reduction of CRP. Nevertheless, there was no significant distinction in CRP levels between RA rats treated with each of the medicinal treatment regimens (P=0.11; Figure 3). The results pointed out that following the stimulation of splenic lymphocytes with collagen, the production and release of inflammatory cytokines, such as IFN-γ and IL-17, increased in the supernatant obtained from splenic cell culture (P<0.05; Figure 3). However, the methotrexate treatment group showed a further decrease in the levels of these two cytokines than naringenin, as compared to the control group (P<0.05; Figure 3).
Figure 3.
The level of inflammatory cytokines in the splenocytes. The values were presented as mean ±SD (&p<0.05 versus healthy rats, *p<0.05 versus PBS-treated RA rats; #p<0.05 versus methotrexate treated RA rats). (Naren. Naringenin-treated RA group; Met., methotrexate-treated RA group).
4. Discussion
The treatment of autoimmune diseases, such as RA, is usually started after the onset of the disease with the emergence of clinical signs and symptoms. Moreover, inducted animals are treated after the observation of joint inflammation signs. The naringenin-treated group demonstrated a significant reduction in the severity of edema and swelling of the paw, in comparison with the methotrexate-treated group. Methotrexate is one of the most effective and well-known immunosuppressive drugs commonly used for the treatment of RA ( 23 ).
Although methotrexate is a relatively useful compound, it has hazardous side effects. The most common complications include pulmonary fibrosis, memory loss, kidney failure, dangerous infections, and injuries to the central nervous system due to a weakened immune system, along with such symptoms as gastrointestinal disorders, mouth ulcers, skin lesions, fatigue, and headache ( 23 , 24 ). Controlling rampant inflammation plays an essential role in the management of autoimmune diseases.
One of the most important causes of tissue damage in inflammatory processes is the occurrence of stress triggered by free radicals ( 18 ). One of the most important justifications for the use of medicinal compounds of natural origin is their significant role in combating the damage caused by the oxygen and nitrogen free radicals. Nowadays, people who eat small amounts of a plant-based dietare more prone to oxidative stress ( 25 ).
In this regard, naringenin as an oral flavonoid has been demonstrated to reduce oxidative stress by increasing the antioxidant systems of superoxide dismutase, catalase, and glutathione in chronic diseases, such as cardiovascular disorder, neurodegenerative, diabetes, cancer, and nephropathy ( 26 ). In vitro, the ability of neutrophils to produce oxygen free radicals has also been demonstrated ( 27 ). The present research also revealed that in terms of improving the total antioxidant capacity of serum, naringenin performed far better than methotrexate due to its potent antioxidant effects.
Since there is no report on the direct antioxidant effect of methotrexate, slight improvements in treated rats can be attributed to its anti-inflammatory effects. Myeloperoxidase (MPO) is a well-known lysosomal enzyme that is stored in the primary granules of neutrophils and depleted when stimulated. It is one of the main enzymes in the production of active oxygen mediators and oxidative damage in inflammatory sites. It has been clearly demonstrated that there is a logical and positive relationship between the severity of the RA disease and the level of serum activity of the MPO enzyme ( 28 ).
The findings of the present study also indicated that the serum level of this enzyme is significantly increased in rats with RA. Due to direct antioxidant properties, naringenin treatment was more effective in the reduction of serum myeloperoxidase activity, as compared to methotrexate. Decreased activity of MPO enzyme in naringenin-treated neutrophils has also been shown in the past ( 27 ).
Due to the activation of the nitric oxide synthase (iNOS) pathway, an increase in the serum level of serum nitric oxide has been reported in RA disease. Nitric oxide is one of the most important causes of nitrate damage ( 29 ). In this regard, a close relationship has been reported between serum nitric oxide levels and disease severity in patients with RA ( 20 ). The results of the current study also pointed to an increase in serum nitric oxide levels in rats with RA. Moreover, treatment with naringenin effectively reduced the serum nitric oxide activity in inducted animals.
It has been demonstrated that naringenin reduces nitric oxide production in lipopolysaccharide-treated macrophage cells by decreasing the expression of iNOS transcription factors ( 30 ). It is worth noting that apart from direct antioxidant effects, naringenin also has a strong direct anti-inflammatory capability, just like common anti-inflammatory drugs, which will be discussed below. For instance, naringenin has been shown to reduce prostaglandin E2 (PGE2) production in macrophage and glial cells treated with lipopolysaccharide by reducing the expression of COX-2 transcription factors ( 30 ).
The CRP, an acute-phase protein, could reflect different pathological processes driven by the underlining acute and chronic inflammation. This factor is an important indicator in the RA tracking; therefore, the reduction of its level is a good predictor ( 31 ). The obtained results of this study indicated that both treatment regimens could similarly reduce the CRP level.
Previous studies have detected a marked decrease in the release of inflammatory mediators, such as IL-1, TNF-α, and monocyte chemotactic protein (MCP-1) in lipopolysaccharide-stimulated microglial cells following treatment with naringenin by inhibiting NF-κb transcription factors and MAP kinase ( 32 ). The results of the current study pointed to a significant reduction in inflammation and arthritis of rats treated with naringenin. The polarization of T lymphocyte responses and production of pro-inflammatory cytokines surely contribute to inflammation and joint destruction in RA ( 9 , 10 ).
The inhibitory effects of naringenin on the production of inflammatory cytokines, major actors in RA produced by T lymphocytes and macrophages, have been demonstrated in recent studies. It has been illustrated that naringenin reduces the production of inflammatory cytokines by macrophages and T lymphocytes by interfering with the Toll-like receptor (TLR)-mediated signaling pathway, altering the stability of cytokine mRNA or their translation. Naringenin increases the degradation of intracellular cytokines by increasing lysozyme degradation ( 16 ).
The effects of naringenin on the polarization of immune responses have been demonstrated in models of autoimmune patients with physiopathological similarities to RA. For example, in experimental autoimmune encephalomyelitis (EAE, experimental model of multiple sclerosis), oral administration of naringenin exacerbated the symptoms by the reduction of immune cell infiltration and the rate of demyelination in the spinal cord tissue. Based on the results, naringenin decreases the lymphocyte subtypes of Th1, Th9, and Th17 by reducing the expression of the main factors involvedin polarization, leading to Th2 lymphocytes polarization.
Decreasing the ratio of immune regulatory lymphocytes (Treg) to the number of inflammatory lymphocytes is one of the effective factors in the creation of autoimmunity ( 33 ). A study on 25 oral flavonoids has suggested that only naringenin can induce Treg cell production by affecting aryl hydrocarbon receptors ( 34 ). In the present study, it was observed that naringin decreased the levels of interferon-gamma (Th1 cell index cytokine) and interleukin 17 (Th17 cell index cytokine) by modulating and polarizing the Th1 and Th17 responses to Th2 and Treg. Therefore, it can be effective in the reduction of RA symptoms in rats ( 33 ).
It has been pointed out that naringenin inhibits immunopathological responses by reducing the frequency of Th1 and Th17 cells without changing the frequency of Th2 cells. In general, the beneficial effects observed by naringenin in this study may be ascribed to its antioxidant properties, along with alterations in the polarization pathway of immune responses. In fact, the current study was a preliminary report, and it is necessary to perform further research in the future, especially with an emphasis on the histopathological and radiological evaluations and assessing the extent of tissue repair in the affected rat joints.
Authors' Contribution
Study concept and design: S. M. A. F.
Acquisition of data: A. H.
Analysis and interpretation of data: S. M. A. F., A. A. T., S. A., and S. R. B. H.
Drafting of the manuscript: S. M. A. F. and A. H.
Critical revision of the manuscript for important intellectual content: S. M. A. F., A. A. T., and S. A.
Statistical analysis: S. M. A. F. and A. H.
Administrative, technical, and material support: S. M. A. F. and S. R. B. H.
Study supervision: S. M. A. F. and A. A. T.
Ethics
All study procedures were approved by the Ethics Committee for Animal Care and Useat Urmia University and Razi Vaccine & Serum Research Institute (Approval No.RVSRI.REC.98.015).
Conflict of Interest
The authors declare that they have no conflict of interest.
Acknowledgement
The present study was sponsored by Urmia University, Urmia, Iran. The authors' deepest appreciation goes to Razi Vaccine and Serum Research Institute for helping us in this research project.
References
- 1.Dai Q, Di Zhou LX, Song XJDD. Development, Therapy. Curcumin alleviates rheumatoid arthritis-induced inflammation and synovial hyperplasia by targeting mTOR pathway in rats. Drug Des Devel Ther. 2018;12:4095. doi: 10.2147/DDDT.S175763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heuser W. Canine rheumatoid arthritis. Can Vet J. 1980;21(11):314. [PMC free article] [PubMed] [Google Scholar]
- 3.Okamoto H, Koizumi K, Kamitsuji S, Inoue E, Hara M, Tomatsu T, et al. Beneficial action of statins in patients with rheumatoid arthritis in a large observational cohort. J Rheumatol. 2007;34(5):964–8. [PubMed] [Google Scholar]
- 4.Pan T, Cheng T-f, Jia Y-r, Li P, Li FJJoe. Anti-rheumatoid arthritis effects of traditional Chinese herb couple in adjuvant-induced arthritis in rats. J Ethnopharmacol. 2017;205:1–7. doi: 10.1016/j.jep.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Shaughnessy ML, Sample SJ, Abicht C, Heaton C, Muir P. Clinical features and pathological joint changes in dogs with erosive immune-mediated polyarthritis: 13 cases (2004–2012) J Am Vet Med A. 2016;249(10):1156–64. doi: 10.2460/javma.249.10.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada Y, Eyre S, Suzuki A, Kochi Y, Yamamoto KJAotrd. Genetics of rheumatoid arthritis: 2018 status. Ann Rheum Dis. 2019;78(4):446–53. doi: 10.1136/annrheumdis-2018-213678. [DOI] [PubMed] [Google Scholar]
- 7.Georgiadis AN, Voulgari PV, Argyropoulou MI, Alamanos Y, Elisaf M, Tselepis AD, et al, editors. Early treatment reduces the cardiovascular risk factors in newly diagnosed rheumatoid arthritis patients. Seminars in arthritis and rheumatism; 2008: Elsevier. doi: 10.1016/j.semarthrit.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Inglis JJ, Šimelyte E, McCann FE, Criado G, Williams ROJNp. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3(4):612. doi: 10.1038/nprot.2008.19. [DOI] [PubMed] [Google Scholar]
- 9.Ghaffary EM, Froushani SMAJLS. Immunomodulatory benefits of mesenchymal stem cells treated with Caffeine in adjuvant-induced arthritis. J Life Sci. 2020;246:117420. doi: 10.1016/j.lfs.2020.117420. [DOI] [PubMed] [Google Scholar]
- 10.Golbahari S, Froushani SMAJLS. Synergistic benefits of Nicotine and Thymol in alleviating experimental rheumatoid arthritis. J Life Sci. 2019;239:117037. doi: 10.1016/j.lfs.2019.117037. [DOI] [PubMed] [Google Scholar]
- 11.Dou W, Zhang J, Sun A, Zhang E, Ding L, Mukherjee S, et al. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br J Nutr. 2013;110(4):599–608. doi: 10.1017/S0007114512005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan N, Wen L, Peng R, Li H, Liu H, Peng H, et al. Naringenin ameliorated kidney injury through Let-7a/TGFBR1 signaling in diabetic nephropathy. J Diabetes Res. 2016;2016 doi: 10.1155/2016/8738760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravishankar D, Rajora AK, Greco F, Osborn HMJTijob, biology c. Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol. 2013;45(12):2821–31. doi: 10.1016/j.biocel.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Alam M, Kauter K, Brown LJN. Naringin improves diet-induced cardiovascular dysfunction and obesity in high carbohydrate, high fat diet-fed rats. Nutrients. 2013;5(3):637–50. doi: 10.3390/nu5030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du G, Jin L, Han X, Song Z, Zhang H, Liang WJCr. Naringenin: a potential immunomodulator for inhibiting lung fibrosis and metastasis. Cancer Res. 2009;69(7):3205–12. doi: 10.1158/0008-5472.CAN-08-3393. [DOI] [PubMed] [Google Scholar]
- 16.Jin L, Zeng W, Zhang F, Zhang C, Liang WJTJoI. Naringenin ameliorates acute inflammation by regulating intracellular cytokine degradation. J Immunol. 2017;199(10):3466–77. doi: 10.4049/jimmunol.1602016. [DOI] [PubMed] [Google Scholar]
- 17.Mulvihill EE, Burke AC, Huff MWJAron. Citrus flavonoids as regulators of lipoprotein metabolism and atherosclerosis. Annu Rev Nutr. 2016;36:275–99. doi: 10.1146/annurev-nutr-071715-050718. [DOI] [PubMed] [Google Scholar]
- 18.Naji Zavareh E, Abtahi Froushani SMJAd. The effects of Cerium oxide nanoparticles on the rat model of rheumatoid arthritis. Armaghane Danesh. 2019;24(5):763–778. [Google Scholar]
- 19.Salehi B, Fokou PVT, Sharifi-Rad M, Zucca P, Pezzani R, Martins N, et al. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals. 2019;12(1):11. doi: 10.3390/ph12010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jahan Tigh M, Abtahi Froshani S, Afzal Ahangaran NJAd. Effect of mesenchymal stem cells treated with 17β-estradiol on the pattern of intrinsic immunity responses in collagen-induced rheumatoid arthritis in wistar rats. Armaghane danesh. 2018;23(1):42–56. [Google Scholar]
- 21.Abtahi Froushani SM, Mashhouri SJZJoRiMS. The Effect of Mesenchymal Stem Cells Pulsed with 17 Beta-Estradiol in an Ameliorating Rat Model of Ulcerative Colitis. Zahedan J Res Med Sci. 2019;21(4) [Google Scholar]
- 22.Hadžović-Džuvo A, Lepara O, Valjevac A, Avdagić N, Hasić S, Kiseljaković E, et al. Serum total antioxidant capacity in patients with multiple sclerosis. Bosn J Basic Med Sci. 2011;11(1):33. doi: 10.17305/bjbms.2011.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herrinton LJ, Woodworth TS, Eworuke E, Amsden LB, Liu L, Wyeth J, et al. Development of an algorithm to detect methotrexate wrong frequency error using computerized health care data. Pharmacoepidemiol Drug Saf. 2019;28(10):1361–8. doi: 10.1002/pds.4858. [DOI] [PubMed] [Google Scholar]
- 24.Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli RJEroci. Methotrexate: an old new drug in autoimmune disease. Expert Rev Clin Immunol. 2014;10(11):1519–30. doi: 10.1586/1744666X.2014.962996. [DOI] [PubMed] [Google Scholar]
- 25.Hafidh R, Abdulamir A, Bakar FA, Abas F, Jahanshiri F, Sekawi ZJAJoP, et al. Antioxidant research in Asia in the period from 2000-2008. Am J Pharmacol Toxicol. 2009;4(3):56–74. [Google Scholar]
- 26.Zaidun NH, Thent ZC, Abd Latiff AJLs. Combating oxidative stress disorders with citrus flavonoid: Naringenin. J Life Sci. 2018;208:111–22. doi: 10.1016/j.lfs.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Cavia‐Saiz M, Busto MD, Pilar‐Izquierdo MC, Ortega N, Perez‐Mateos M, Muñiz PJJotSoF, et al. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. 2010;90(7):1238–44. doi: 10.1002/jsfa.3959. [DOI] [PubMed] [Google Scholar]
- 28.Abtahi Froushani. Investigating the effects of Thymol on animal model of Rheumatoid Arthritis. J Fasa Univ Med Sci. 2018;8(3):890–900. [Google Scholar]
- 29.Mashhouri S, Abtahi Froushani SM, Tehrani AAJIJoMS. Non-Adherent Bone Marrow-Derived Mesenchymal Stem Cells Ameliorate Clinical Manifestations and Inflammation in an Experimental Model of Ulcerative Colitis in Rats. Iran J Med Sci. 2020 doi: 10.30476/ijms.2020.72514.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chao C-L, Weng C-S, Chang N-C, Lin J-S, Kao S-T, Ho F-MJNr. Naringenin more effectively inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in macrophages than in microglia. Nutr Res. 2010;30(12):858–64. doi: 10.1016/j.nutres.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Benito M. Rheumatoid Arthritis: An Outlook of the Main Inflammatory Cells and Mediators Involved, and Treatments to Target Inflammation. Acta Sci Med Sci. 2018;2(9):66–70. [Google Scholar]
- 32.Park HY, Kim G-Y, Choi YHJIjomm. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-κB and inhibiting mitogen-activated protein kinases. Int J Mol Med. 2012;30(1):204–10. doi: 10.3892/ijmm.2012.979. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Niu X, Wu C, Wu DJFii. Naringenin modifies the development of lineage-specific effector CD4+ T cells. Front Immunol. 2018;9:2267. doi: 10.3389/fimmu.2018.02267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang H-K, Yeh C-H, Iwamoto T, Satsu H, Shimizu M, Totsuka MJJoa, et al. Dietary flavonoid naringenin induces regulatory T cells via an aryl hydrocarbon receptor mediated pathway. J Agric Food Chem. 2012;60(9):2171–8. doi: 10.1021/jf204625y. [DOI] [PubMed] [Google Scholar]