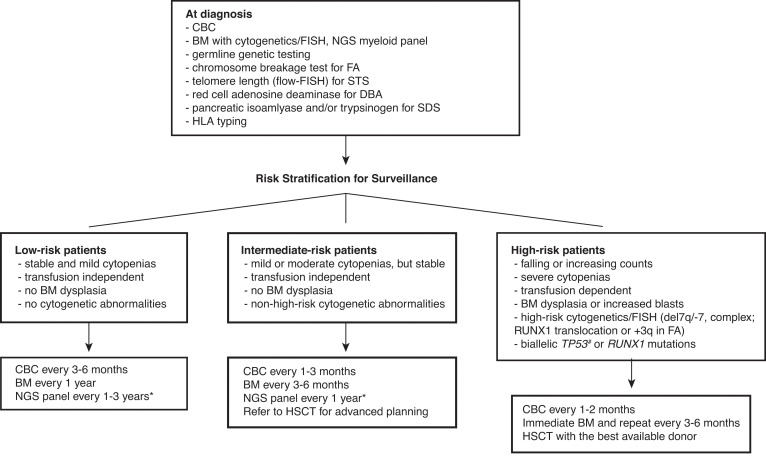
Figure 1.
Surveillance recommendations for patients with IBMFS based on the risk of malignant transformation. Detailed diagnostic workup for BMF is described by DeZern and Churpek.7 Once diagnosed, patients are followed by CBC with differential, BM aspiration and biopsy with cytogenetics, FISH, and NGS myeloid panel at regular intervals. The frequency of follow-up depends on the disease and mutation types, baseline counts and BM findings, and the presence of interval changes on subsequent visits. Although the NGS myeloid panel is not a part of standard practice for the management of IBMFS, it is becoming more widely used with advancement of our understanding in CH in both IBMFS and the general population. NGS myeloid panel results are particularly useful to select patients who are at high risk of malignant transformation without overt clinical signs of disease progression. We can increase the frequency of surveillance and initiate discussion and planning for early intervention for the best possible outcome in these high-risk patients. *The optimal frequency and the best genomic source (PB vs BM) of NGS myeloid panel testing have not been fully established. #Although biallelic TP53 mutations are known high-risk features, conventional NGS techniques do not readily provide allelic status of a given mutation. The severity of BMF is defined as the following: mild—ANC <1500/µL, hemoglobin ≥8 g/dL, or platelets 50-150 K/µL; moderate—ANC <1000/µL, hemoglobin <8 g/dL, or platelets <50 K/µL; severe—ANC <500/µL, hemoglobin <8 g/dL, or platelets <30 K/µL. ANC, absolute neutrophil count; CBC, complete blood count; FISH, fluorescence in situ hybridization; PB, peripheral blood.