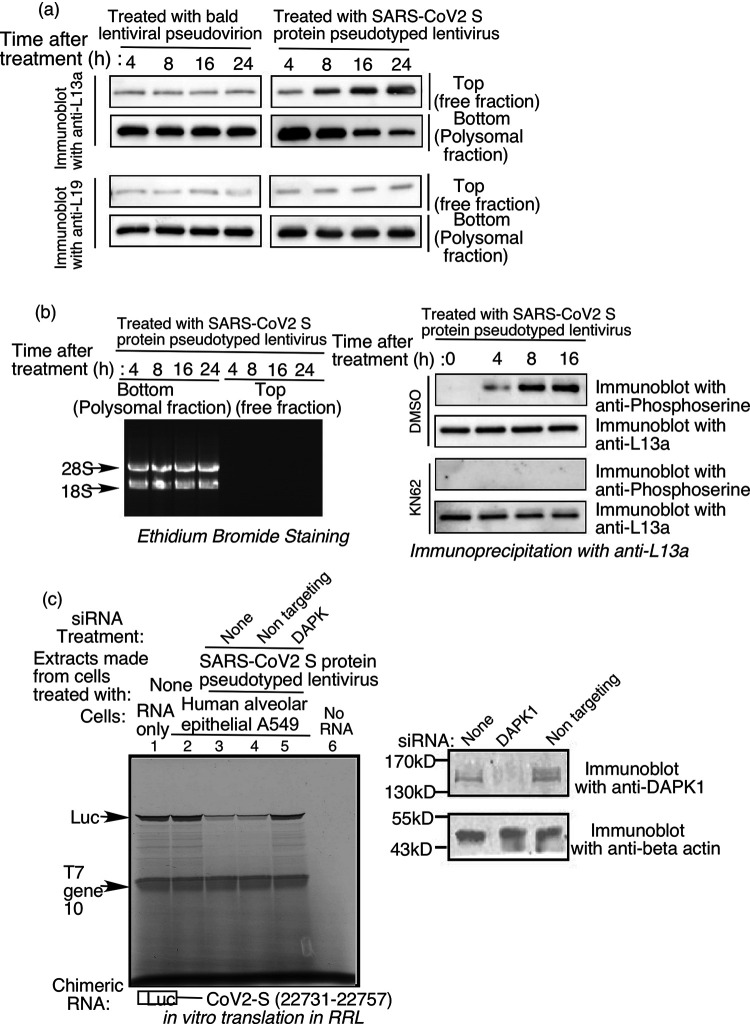
FIG 8.
Treatment of A549 lung cells with SARS-CoV-2 S pseudotyped lentivirus triggers phosphorylation and release of L13a from the ribosome. (a) Detection of ribosome-associated and free L13a in A549 cells exposed to S protein. Extracts from A549 cells treated with “bald” (negative control lacking S protein) or S protein pseudotyped lentivirus for the indicated amounts of time were separated into polysome (bottom) and ribosome-free cytosolic (top) fractions, which were then immunoblotted with anti-L13a or anti-L19 antibodies (Thermofisher #14701-1-AP). (b) (left panel), Confirmation of ribosomal and non-ribosomal fractions used in (a). RNA was extracted from the separated fractions using TRIzol, resolved on an agarose gel, and visualized by staining with ethidium bromide. 8b (right panel), Treatment with SARS-CoV-2 S pseudotyped lentivirus induces DAPK-dependent serine phosphorylation of L13a in A549 cells. A549 cells were pretreated with DAPK inhibitor KN62 (or DMSO solvent as a negative control) for 1 h before incubation with the lentivirus. After the indicated amounts of time, cell extracts were prepared and subjected to immunoprecipitation (IP) with anti-L13a antibody, followed by immunoblotting with anti-phosphoserine or anti-L13a antibodies. (c) Requirement of DAPK1 in SARS-CoV-2 S protein-induced and VAIT element-mediated translation control. A549 cells were either untreated or treated with a DAPK1 specific or a non-targeting (used as negative control) siRNA and the steady state level of DAPK1 and beta actin were monitored by immunoblot analysis with anti-DAPK1 and anti-beta actin antibodies. 48 h after siRNA transfection, cells were incubated with SARS-CoV-2 S protein pseudotyped lentivirus for 24 h. Cell lysates were then prepared and used in translation control assay as described earlier. Results showed DAPK1 knocked down cells failed to inhibit translation from chimeric RNA of luciferase and VAIT element (lanes 3 and 4 vs lane 5).