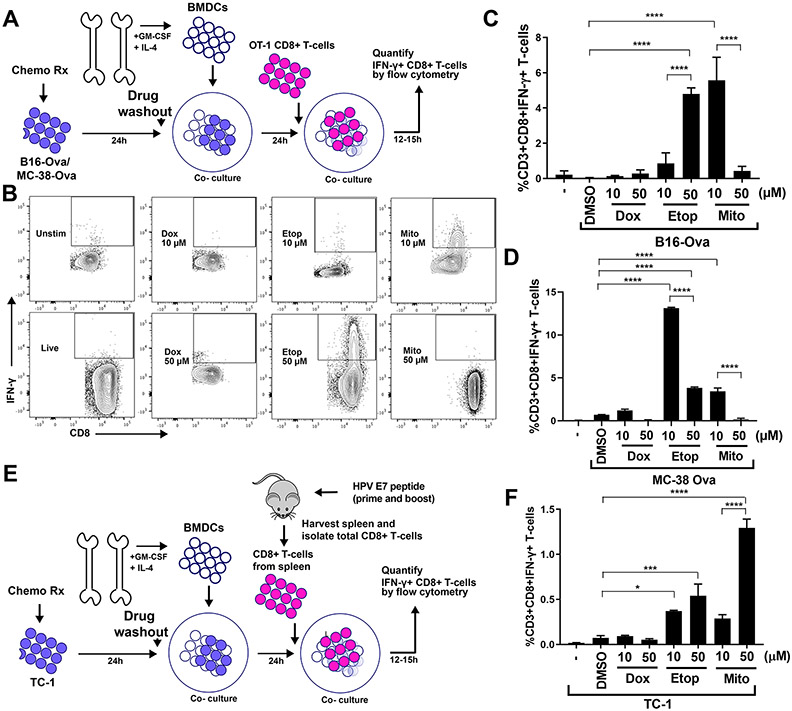
Figure 1: An experimental system to assess DC-mediated T cell IFN-γ responses to genotoxically damaged tumor cells.
(A) Schematic of the in vitro experimental system. B16-Ova or MC-38-Ova cells were treated with DNA-damaging agents for 24 hours, washed and incubated with primary bone marrow-derived dendritic cells (BMDC) for another 24 hours. Following this, OT-I CD8+ T cells expressing a TCR transgene that specifically recognizes the Ova-derived peptide SIINFEKL in the context of H2-Kb (OT-1) (31,32) were added and evaluated for intracellular IFN-γ 15 hours later. (B) T cells resulting from the experimental protocol described in (A) were identified by CD3 staining, and re-gated for IFN-γ and CD8 expression. Representative flow cytometry plots assessing IFN-γ+ CD8+ T cell populations (boxed region) induction by treatment of B16-Ova cells with 10 μM or 50 μM of doxorubicin, etoposide, or mitoxantrone. (C and D) Quantification of BMDC-mediated induction of IFN-γ+ CD8+ T-cells from 5 (C) or 3 (D) independent experiments with B16-Ova and MC-38-Ova cells, respectively, described in (A and B). The first lane marked “-” indicates the percen67age of IFN-γ+ CD8+ T cells produced by co-culture of BMDCs and T cells in the absence of tumor cells. (E) Schematic of the in vitro experimental system using TC-1 cells, treated co-cultured with BMDCs as described in (A), then co-cultured with CD8+ T cells isolated from mice vaccinated (primed and boosted) with an HPV-E7 peptide. As in (A), T cells were then assessed for intracellular IFN-γ 15 hours later. (F) Quantification of IFN-γ+ CD8+ T cells from 3 experiments described in (E). “-” as described for (C and D). For all the above panels, error bars indicate SEM, *P<0.05, ***P<0.005, and ****P<0.0001 by ANOVA followed by Sidak’s multiple comparisons test.