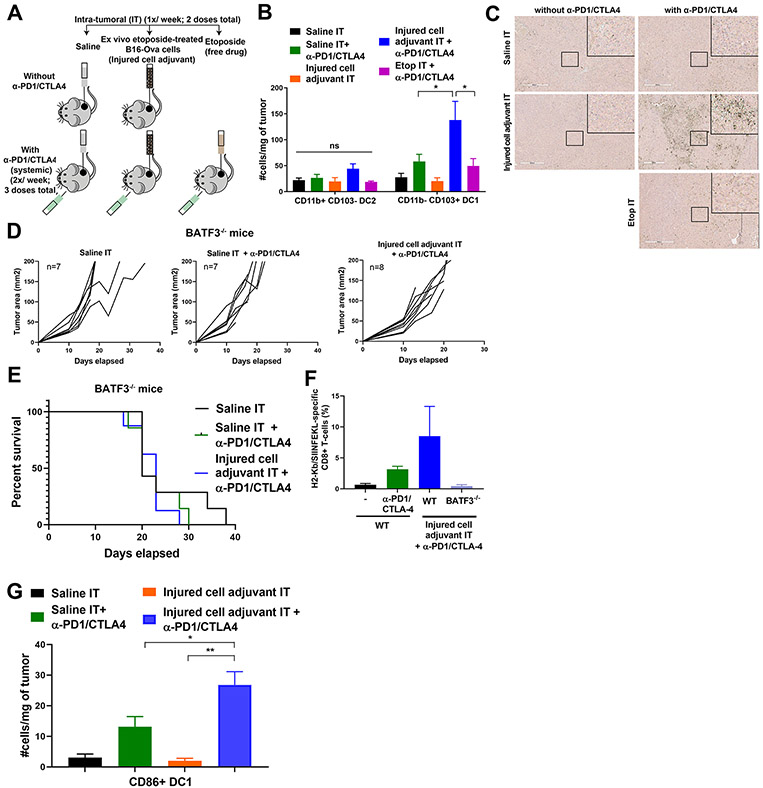
Figure 6: Intra-tumoral administration of the injured cell adjuvant plus systemic ICB increases DC1 density in the tumor and requires BATF3 for efficacy.
(A) Schematic of the experimental design and dosing regimen used to test the effect of intra-tumoral etoposide-treated B16-Ova cells in combination with systemic anti-PD1/CTLA4 on the frequency of intra-tumoral DC. (B) Quantification of intra-tumoral CD11b-CD103+ DC1 and CD11b+CD103− DC2 subsets from treated tumors analyzed by flow cytometry (5 mice per group). Error bars represent SEM. * P<0.05 by ANOVA followed by Sidak’s multiple comparisons test. “ns” indicates P>0.05 by ANOVA. (C) Tumor sections were stained with an antibody to BATF3. Insets show higher-magnification images of the boxed central region of each section. Scale bar, 400 μm. (D) Tumor growth curves of Batf3−/− mice treated with intra-tumoral saline or etoposide-treated B16-Ova cells (injured cell adjuvant) in combination with systemic anti-PD1 and anti-CTLA4 antibodies. “n” indicates the number of mice in each group. (E) Kaplan-Meier survival curves of the experiment in (D). P=0.5220 by log-rank test. (F) Frequency of circulating H2-Kb/SIINFEKL specific CD8+ T cells from WT and Batf3−/− mice treated as indicated. (G) Quantification of intra-tumoral CD86+ DC1 from tumors treated 24 hours prior with one dose of saline or injured cell adjuvant (IT) in presence or absence of one dose of systemic anti-PD1 and anti-CTLA4 48 hours prior. Error bars represent SEM, 5 mice per group. *P<0.05 and **P<0.01 by ANOVA followed by Sidak’s multiple comparisons test.