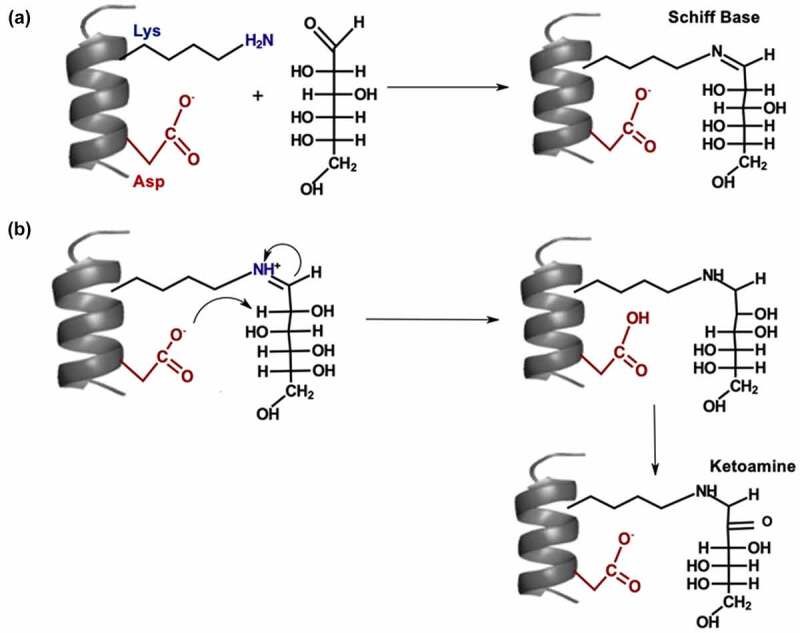
Figure 3.
(a) Mechanism for Schiff base formation. (b) Mechanism for Amadori rearrangement on glycated lysine.
[Alt Text: (A) The side chains of lysine and aspartic are shown within a coil. There is a condensation reaction between lysine side chain and first carbon atom of glucose to form Schiff base. (B) The reaction mechanism starts with nucleophilic attack on the NH+ group of the lysine side chain by the first carbon atom of glucose and a nucleophilic attack on the proton attached to the second carbon by the carboxylate ion of the aspartic acid. Two hydrogen atoms are removed from the second carbon of glucose to form ketoamine.].