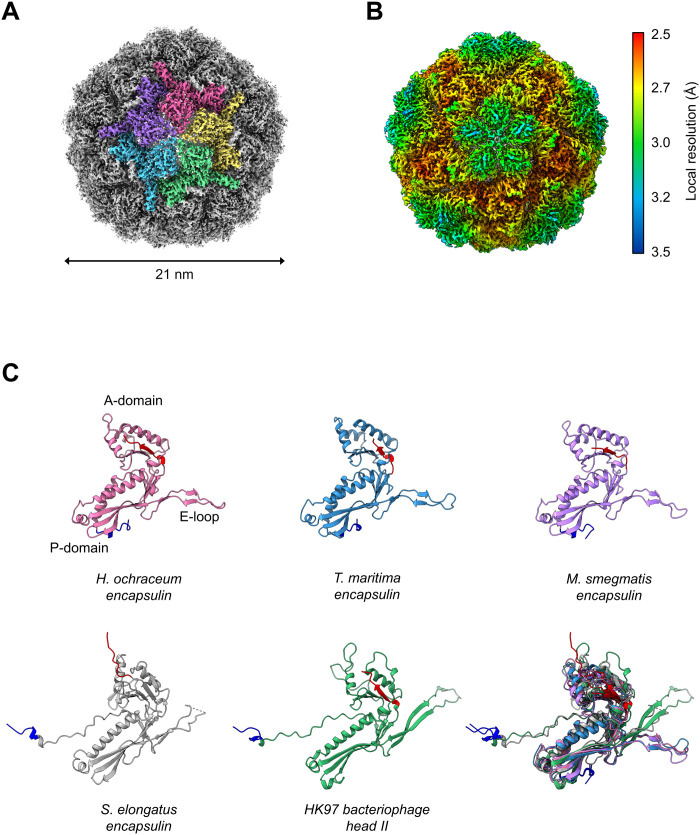
Fig. 2. Architecture of the H. ochraceum encapsulin nanocompartment shell.
Visualization of the electronic potential map of the H. ochraceum encapsulin from an icosahedrally averaged single-particle reconstruction. (A) Exterior of the encapsulin shell visualized at 2.4-Å resolution. Five subunits of the encapsulin nanocompartment shell have been colored to highlight the fivefold axis. (B) Icosahedral EM map of Loaded-Enc sharpened by local resolution estimate and colored by local resolution. The estimated resolution varies across the exterior of the encapsulin nanocompartment with the lowest resolution at the fivefold pores. Color key of resolution mapping is shown on the right-hand side of the figure. (C) Shared phage-like fold in the HK97 bacteriophage capsid and encapsulin proteins. Monomeric subunit of the H. ochraceum encapsulin protein modeled from our reconstruction is shown (pink), with comparisons to other T = 1 encapsulins from T. maritima [blue, Protein Data Bank (PDB) ID: 3DKT], Mycobacterium smegmatis (lilac, PDB ID: 7BOJ), and S. elongatus (gray, PDB ID: 6X8M). The HK97 bacteriophage head II T = 7 monomer (green, PDB ID: 2FT1) is also shown. The N terminus of each monomer is highlighted in blue, and the C terminus is highlighted in red. Bottom right: Overlay comparison of the encapsulin monomers showing similar A- and P-domain orientations.