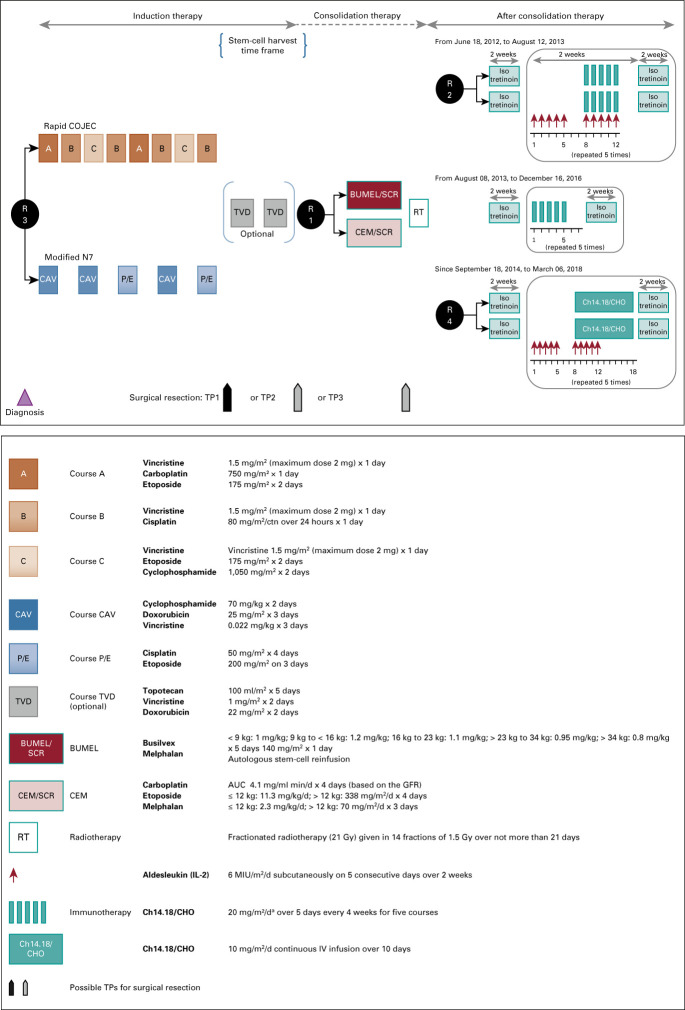
FIG A1.
Treatment flowchart of the HR-NBL1 Protocol (ClinicalTrials.gov: NCT01704716, EudraCT: 2006-001489-17) over the whole period. aInfants and children with a body weight below 12 kg will be dosed at 0.67 mg/kg/d. In infants weighing ≤ 5 kg, a further 1/3 dose reduction is advised. AUC, area under the curve; BUMEL, busulfan and melphalan; CAV, cyclophosphamide plus doxorubicin or vincristine; CEM, carboplatin, etoposide, and melphalan; CH14.18/CHO, human-mouse chimeric monoclonal anti-disialoganglioside GD2 antibody ch14.18 produced in Chinese hamster ovary (CHO) cells; COJEC, chemotherapy schedule COJEC defined below; GFR, glomerular filtration rate; IL-2, interleukin-2; IV, intravenous; P or E, cisplatin or etoposide; R1, randomization 1; R2, randomization 2; R3, randomization 3; R4, randomization 4; RT, radiotherapy; SCR, stringent complete response; TP, time period; TVD, topotecan-vincristine-doxorubicin.