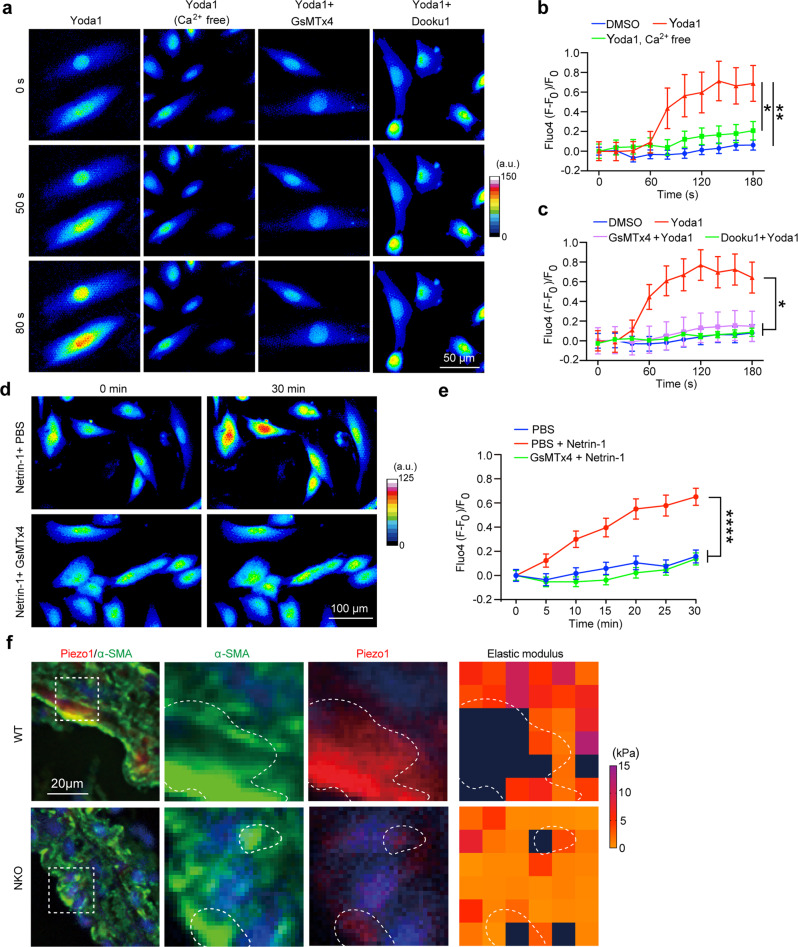
Fig. 7. Netrin-1 regulates intracellular Ca2+ influx via Piezo1 in VSMC.
a Representative images of intracellular Ca2+ signal in VSMC treated with reagents and time period as indicated. The signal intensity scale is indicated on the right. a.u., arbitrary unit. b, c Time course quantification of Ca2+ flux in VSMC treated as indicated. For b, n = 5, 8, and 5 independent measurements for DMSO, Yoda1 Ca2+ free, and Yoda1, respectively. One-way ANOVA followed by uncorrected Fisher’s LSD test. *P = 0.025 and **P = 0.0082. For c, n = 5, 5, 8, and 11 independent measurements for DMSO, Yoda1, GsMTx4 +Yoda1, and Dooku1 + Yoda1, respectively. One-way ANOVA followed by Turkey’s multiple comparisons test. *P = 0.0191 (DMSO vs Yoda1), *P = 0.0230 (Yoda1 vs GsMTx4 +Yoda1), and *P = 0.0321 (Yoda1 vs Dooku1 +Yoda1). Data are represented as mean values ± SEM and multiple comparisions tests are performed at t = 180 s for both b and c. d, e Representative images (d) and quantification (e) of Ca2+ levels in VSMC were treated as indicated. The signal intensity scale is indicated on the right of d. a.u., arbitrary unit. For e, n = 33, 24, and 26 cells for PBS, PBS + Netrin-1, and GsMTx4 + Netrin-1, respectively. One-way ANOVA followed by Turkey’s multiple comparisons test. ****P < 0.0001. Data are represented as mean values ± SEM. f IF staining of α-SMA (green) and Piezo1 (red) in aortic sections of WT or NKO mice. The dashed box indicates the magnified area of AFM and immunostaining. Piezo1 and α-SMA expression and elastic modulus in areas of interest are outlined. Nuclei are stained with DAPI (blue). Tissue sections in f were transversal sections. Source data are provided as a Source Data file.