Figure 2. GlcNDAz is incorporated into N-glycans.
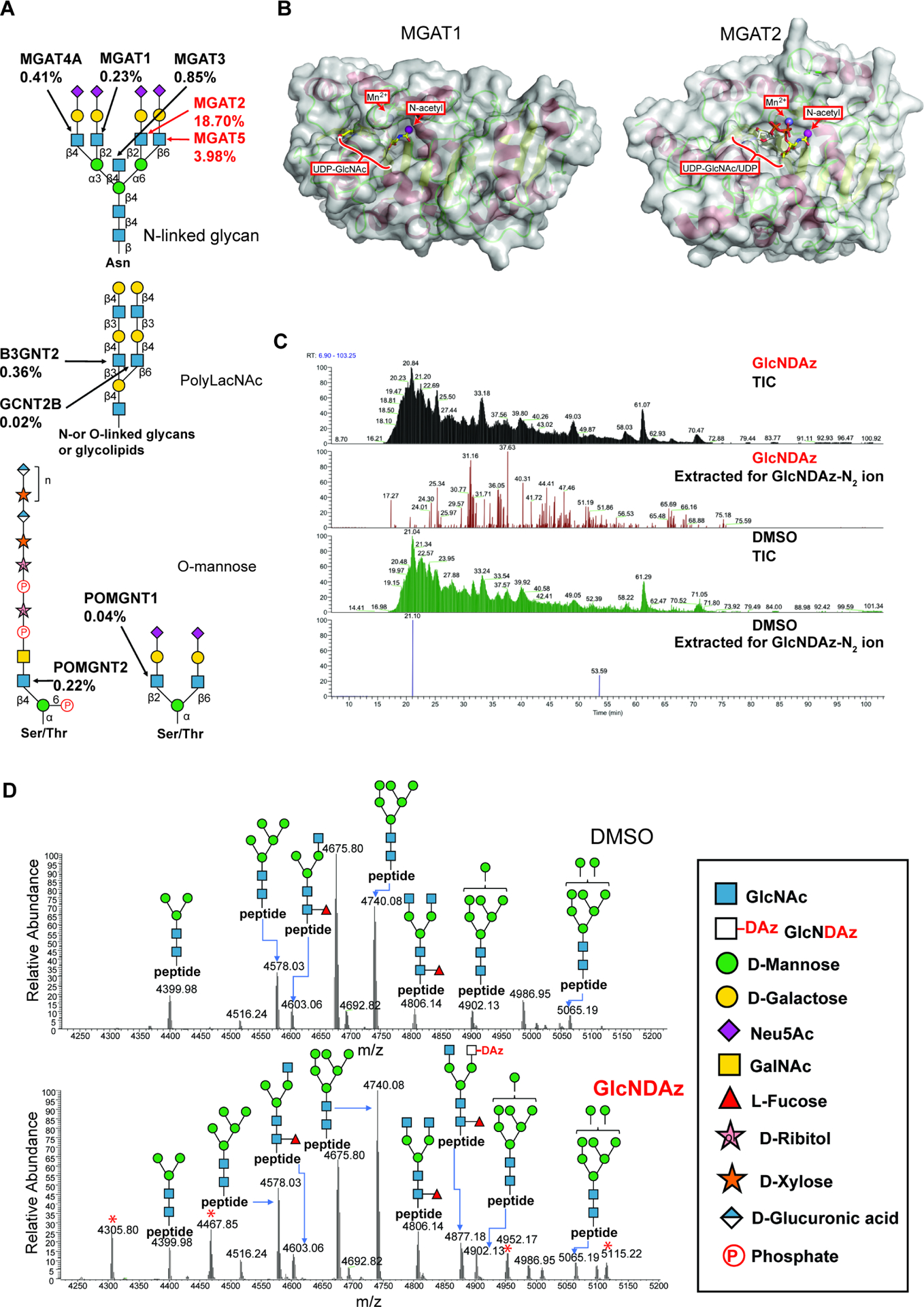
(A) Biosynthetic roles of GlcNAc-transferases were examined using recombinant Golgi GlcNAc-transferases (Fig. S2). Percentages represent relative activity of GlcNAc transferases in using UDP-GlcNDAz as a substrate compared to UDP-GlcNAc. Percentages are averaged from two trials. (B) Modeled structures of human MGAT1 and MGAT2 (PDB 5VCM) (Kadirvelraj et al., 2018) in complex with UDP-GlcNAc. The human MGAT1 structure was modeled based on the structure of the rabbit MGAT1:UDP-GlcNAc:Mn2+ (PDB 1FOA). (Unligil et al., 2000) A magenta sphere marks the methyl group to which the diazirine is attached in GlcNDAz. (C) Glycopeptides from T84 UAP1(F383G) cells treated with Ac3GlcNDAz-1P(AcSATE)2 or DMSO were analyzed by mass spectrometry, and ion chromatograms were extracted for the GlcNDAz – N2 ion. Chromatograms are from single trial. (D) Comparison of glycoforms detected on peptide 38VVRPDSELGERPPEDNQSFQYDHEAFLGK66 from human reticulocalbin-1 from T84 UAP1(F383G) cells treated with Ac3GlcNDAz-1P(AcSATE)2 or vehicle. Red asterisks label peaks (m/z 4305.80, 4467.85, 4952.17, 5115.22) assigned to other co-eluting glycopeptides. Spectrum is from single trial. See also Fig. S2, Fig. S3, Fig. S4, and Data S1.
