Abstract
Background:
Cancer survivors develop other chronic medical conditions due to shared risk factors and the delayed effects from cancer treatment. We investigated the trends in the prevalence and estimated the population sizes of chronic diseases among adult cancer survivors in the US from 2002 to 2018.
Methods:
Using the 2002–2018 National Health Interview Survey data, we calculated the age-sex-race/ethnicity-adjusted prevalence and estimated the population sizes of the following chronic conditions among cancer survivors: hypertension, diabetes, stroke, heart disease, chronic obstructive pulmonary disease (COPD)/asthma, hepatitis, arthritis, liver disease, kidney disease, and morbid obesity. We also examined multiple chronic conditions (MCC, three or more health conditions). The trends for MCC were further examined by sociodemographic factors to identify high-risk populations. Parallel analyses were performed for participants without a cancer history to provide a reference.
Results:
Among 30,728 cancers survivors, we observed increasing trends in prevalence of hypertension, diabetes, kidney disease, liver disease and morbid obesity, while decreasing prevalence trends of ischemic heart disease, COPD, and hepatitis. Cancer survivors with MCC increased from 4.7 million in 2002 to 8.1 million in 2018 (prevalence from 48.7% to 53.0%). The increase was more pronounced among survivors aged 18–44 years. Among adults without a cancer history, MCC prevalence also increased, although slower than among survivors.
Conclusions:
The number of adult cancer survivors in the US with comorbid illnesses has increased substantially over the past two decades. Optimal management of comorbid conditions and aggressive interventions for risk reduction may benefit the cancer survivor population.
Keywords: Cancer survivorship, Comorbidity, Disease burden, Public Health, Multimorbidity management
Precis for use in the Table of Contents:
The prevalence of most comorbid illnesses increased significantly amongst cancer survivors in the United States. Cancer survivors with multiple comorbidities almost doubled from 2002 to 2018.
Introduction
Due to improvements in early diagnosis and advances in cancer treatment, the number of cancer survivors grew rapidly from 10 million in 2002 to 16.9 million in 2019, and is projected to reach 22.1 million by 2031.1,2 Cancer survivors commonly suffer from comorbid chronic conditions due to shared risk factors such as smoking and obesity in addition to lasting and delayed effects of cancer treatment.2–4 The presence of comorbid chronic conditions has been linked to psychological distress, increased healthcare service use, higher medical costs, adverse clinical outcomes, and financial hardship in cancer survivors.5–10
Management of cancer and comorbid conditions has been challenging. If the condition developed prior to cancer diagnosis, it can frequently complicate or alter cancer treatment, and can lead to inferior outcomes for cancer patients undergoing active treatment.9,11 On the other hand, if the condition developed after cancer diagnosis, its management is often compromised in consideration of patients’ cancer history.9,11 To assist clinicians navigating some of these challenges, The American Society of Clinical Oncology published a curriculum for cancer survivorship and emphasized the need for management of comorbid conditions as the cancer survivor population continues to grow rapidly.12
Understanding the current burden of comorbid chronic conditions among cancer survivors is essential for care planning and delivery. For example, despite decreased smoking rates and improved cancer treatment outcomes during the last two decades in the US, obesity and metabolic syndromes have continued to increase dramatically. The changes in these lifestyle and clinical factors may have affected the type and burden of comorbid conditions in cancer survivors in the U.S.13,14 However, to our knowledge, the trend of chronic conditions among cancer survivors has never been firmly elucidated in the current literature, limiting effective care planning and resource allocation. Using a nationally representative sample, this study examines the prevalence and population size of common chronic conditions and multiple chronic conditions (MCC) among cancer survivors over the past two decades in the US in comparison to their peers without a cancer history.
Methods
Study Participants
We conducted a population-based study using the National Health Interview Survey (NHIS) from 2002 through 2018. The NHIS is an annual, cross-sectional, national health survey conducted by the National Center for Health Statistics, Centers for Disease Control and Prevention.15 The survey collects information on health of the civilian, noninstitutionalized, household population of the United States.16 Two sets of questionnaires were used since 1997 including 1) the Core Questionnaire collecting basic health and demographic information from all individuals living in a household and 2) the Sample Questionnaire collecting detailed health information for one adult and one child, each randomly selected from the household. To be consistent in measured chronic conditions, we chose 2002 as the first year of our analysis, because it was when NHIS started to collect information about arthritis, lupus, fibromyalgia, and other rheumatologic diseases.
The annual response rate of NHIS is approximately 80 percent of the eligible households in the sample.16 In this analysis, we included adult participants who responded to both questionnaires. Individuals were excluded if they did not answer the questions on any of their chronic conditions or basic sociodemographic information (age, sex, race/ethnicity, education level and insurance status), reported a cancer diagnosis prior to age 18 years or at unknown age, or were diagnosed with only non-melanoma skin cancer (Figure 1). We included both cancer survivors and individuals without a history of cancer to compare the difference in trends over time. This study was exempt from IRB review by the Office of Research Subject Protection at the Roswell Park Comprehensive Cancer Center because the NHIS data are de-identified and publicly available.
Figure 1.
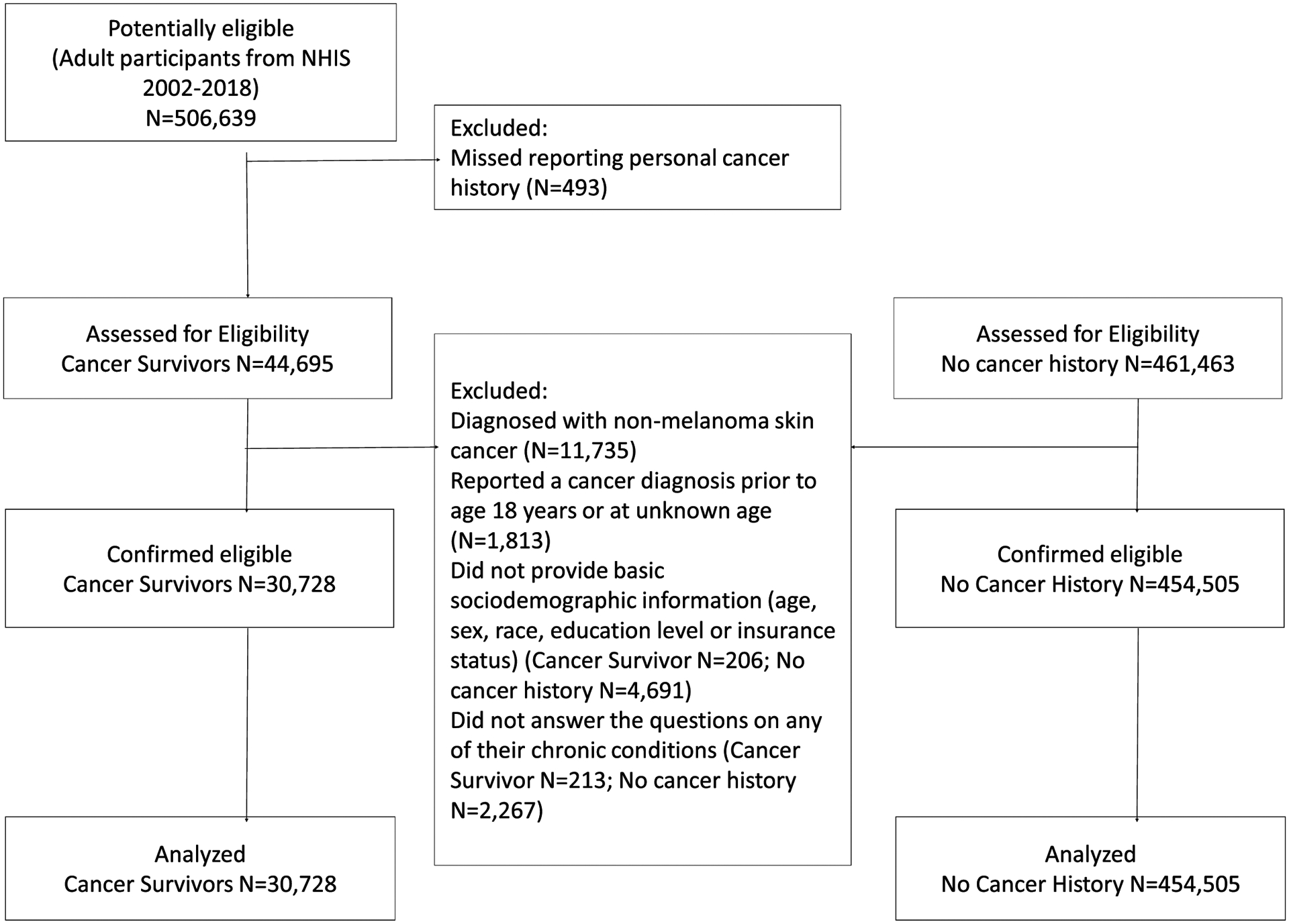
Flowchart of the study population inclusion/exclusion and analyses.
Cancer History
Participants were asked whether a physician had told them that they had cancer or a malignancy, and, if yes, what type of cancer and when the cancer was diagnosed. Cancer survivors were defined as participants reporting any history of cancer, consistent with the National Cancer Institute (NCI) and the National Coalition for Cancer Survivorship.17,18
Chronic conditions
Participants reported whether they had ever been told by a doctor or health care provider that they had hypertension, diabetes, stroke, ischemic heart disease (angina pectoris, coronary heart disease, heart attack), other heart diseases, chronic obstructive pulmonary disease (COPD), hepatitis, or arthritis. They also reported whether they had been diagnosed with any of the following conditions in the past 12 months: asthma attack, weak or failing kidneys, or any liver disease. Hypertension was determined by whether participants were told they had hypertension or high blood pressure on two or more doctor visits. Insulin-dependent diabetes was defined as diabetes managed with insulin. Individuals who reported COPD or active asthma were categorized as having lung diseases. Morbid obesity was defined as body mass index (BMI) >40 kg/m2 or BMI >35 kg/m2 with one or more comorbid conditions, where BMI was calculated with self-reported height and weight.19 MCC was defined as having three or more chronic conditions.
Sociodemographic information
Participants also reported information on sex, age, educational attainment, race/ethnicity, marital status, poverty status, current smoking status, and health insurance coverage.
Statistical Analyses
To characterize population characteristics over time, survey participants were grouped into six periods across the survey years (2002–2003, 2004–2006, 2007–2009, 2010–2012, 2013–2015, 2016–2018). Cochran-Armitage trend tests were used to determine whether demographic and health characteristics of the population changed over time. To examine prevalence of chronic conditions, we used multivariable linear probability regression to estimate the age, sex, and race/ethnicity adjusted-prevalence of cancer survivors with each condition and MCC from 2002 to 2018 and plotted the prevalence against time. Average annual change in prevalence of each condition and MCC was calculated with the survey year entered as a continuous variable in the models. The weighted population sizes of cancer survivors with each condition and MCC were estimated based on the weighted number of cancer survivors and the weighted prevalence of MCC in the nationally representative NHIS. The estimated population sizes were plotted against time to visualize the disease burden over the study period. Stratified analyses were conducted for MCC by age group, sex, race/ethnicity and insurance. In addition, we tested interaction between year and each sociodemographic factor to explore subgroups of cancer survivors with the most rapidly increasing trend of MCC.
Parallel analyses were also performed for adults without a cancer history in the US from 2002–2018 for reference. The difference in trends of adjusted prevalence of each condition and MCC between cancer survivors and individuals without a cancer history was tested by including an interaction term of cancer status and survey year in the models.
All analyses were conducted using SAS statistical software, version 9.4 (SAS Institute Inc). The SAS procedures PROC SURVEYFREQ and PROC SURVEYREG were used for descriptive analyses and modeling. Both procedures incorporate the complex survey sample design of NHIS, including stratification, clustering, and unequal weighting. The variances of the regression parameters were computed using the Taylor series (linearization) method to estimate the sampling errors of estimators based on the complex sample design. All statistical significance testing was 2-sided at p<0.05. All estimates were weighted to account for the complex design and nonresponse of the NHIS according to the recommendations from the National Center for Health Statistics.15
Results
Participant Characteristics
The characteristics of 30,728 adult cancer survivors are shown in Table 1 by NHIS survey period. The proportion of adult cancer survivors increased steadily from 4.8% in 2002–2003 to 6.5% in 2016–2018. Significant upward trends were seen for all characteristics including older age, male sex, high education level, public insurance, and history of two or more cancers among cancer survivors since 2002 (all p<0.01).
Table 1.
Sample Characteristics of Cancer Survivors by Period
| 2002–2003 | 2004–2006 | 2007–2009 | 2010–2012 | 2013–2015 | 2016–2018 | ||
|---|---|---|---|---|---|---|---|
| Sample sizea | 3,056 | 4,613 | 4,121 | 5,773 | 6,754 | 6,411 | |
| Weighted population size, 1,000sb | 9,630 | 10,805 | 11,668 | 13,024 | 13,599 | 15,353 | |
| Age (years), %c | |||||||
| 18 to 44 | 14.6 | 15.3 | 11.9 | 11.7 | 10.3 | 9.6 | |
| 45 to 54 | 16.1 | 15.8 | 16.5 | 14.9 | 12.3 | 12.7 | |
| 45 to 64 | 20.5 | 21.1 | 23.3 | 23.9 | 24.8 | 23.4 | |
| 65 to 74 | 23.5 | 21.7 | 23.6 | 24.2 | 26.7 | 28.4 | |
| 75+ | 25.2 | 26.1 | 24.6 | 25.4 | 25.9 | 25.9 | |
| Female, % | 62.4 | 61.3 | 62.3 | 59.5 | 60.0 | 59.7 | |
| Education, % | |||||||
| Less than High School | 19.7 | 18.6 | 16.5 | 13.9 | 13.5 | 11.7 | |
| High School/GED | 31.7 | 30.5 | 30.1 | 29.1 | 29.0 | 25.6 | |
| More than High School | 48.6 | 50.8 | 53.4 | 57.0 | 57.5 | 62.8 | |
| Race, % | |||||||
| Non-Hispanic White | 86.7 | 85.2 | 82.8 | 82.2 | 81.1 | 80.1 | |
| Hispanic | 4.5 | 5.1 | 6.1 | 5.8 | 7.2 | 7.2 | |
| Non-Hispanic Black | 6.6 | 7.2 | 7.8 | 8.8 | 8.7 | 8.5 | |
| Non-Hispanic Other | 2.2 | 2.5 | 3.3 | 3.2 | 2.9 | 4.2 | |
| Married, % | 59.3 | 60.5 | 58.9 | 57.8 | 58.2 | 59.1 | |
| Insurance, % | |||||||
| Private Insurance | 69.6 | 66.7 | 66.1 | 61.1 | 58.5 | 60.7 | |
| Public Insuranced | 24.2 | 27.1 | 27.0 | 32.8 | 36.9 | 36.1 | |
| Uninsured | 6.2 | 6.2 | 6.8 | 6.1 | 4.6 | 3.2 | |
| Poverty status (FPLe), % | |||||||
| Less than 100 % | 8.3 | 7.7 | 8.8 | 9.3 | 9.6 | 8.2 | |
| 100–199% | 14.1 | 16.7 | 15.6 | 15.7 | 16.6 | 15.2 | |
| 200–399% | 24.5 | 22.5 | 24.5 | 27.6 | 27.9 | 28.0 | |
| 400% or above | 25.8 | 28.6 | 35.9 | 33.6 | 36.1 | 40.6 | |
| Unknown | 27.3 | 24.6 | 15.3 | 13.7 | 9.8 | 7.9 | |
| Current Smoking, % | 19.0 | 17.9 | 17.6 | 16.0 | 14.1 | 12.8 | |
| History of two or more cancers, % | 9.0 | 8.8 | 9.1 | 9.4 | 9.9 | 10.6 |
Cochran-Armitage trend tests showed statistically significant trends in proportions of all sample characteristics (all P < .01).
The estimated size of populations of cancer survivors were estimated using National Health Interview Survey complex survey design and survey weights.
All percentage presented are weighted.
Public insurance included self-reported Medicaid, Medicare, military health care/Veterans Affairs insurance, Medi-Gap, and Indian Health Service insurance.
FPL stands for federal poverty level.
The characteristics of 454,505 individuals without cancer history are shown in Supplement Table S-1.
Trends in Individual Chronic Health Conditions
Table 2 describes the prevalence of chronic conditions among cancer survivors from 2002–2018. The prevalence statistically significantly increased for hypertension (35.9% to 40.6%, p<0.001), diabetes (13.4% to 15.3%, p=0.003), kidney disease (4.2% to 5.0%, p=0.03), liver disease (3.1% to 4.4%, p=0.001) and morbid obesity (5.9% to 9.5%, p<0.001), after adjusting for age, sex, and race/ethnicity. By contrast, the adjusted prevalence decreased significantly for ischemic heart disease (12.3% to 10.6%, p=0.001), COPD (9.4% to 6.9% p<0.001), and hepatitis (5.9% to 4.5%, p=0.04).
Table 2.
Trends in Each Chronic Conditions from 2002 to 2018 in US Adult Cancer Survivors
| Hypertension | 4,062 | 7,730 | 35.90% | 40.60% | 0.296 | <0.001 | 0.22 |
| Diabetes | 1,356 | 2,570 | 13.40% | 15.30% | 0.149 | 0.003 | 0.38 |
| Insulin-dependent diabetes | 413 | 695 | 4.30% | 4.00% | 0.027 | 0.36 | 0.27 |
| Stroke | 555 | 1,263 | 4.90% | 6.30% | 0.052 | 0.14 | 0.30 |
| Heart Disease | 2,481 | 3,767 | 20.60% | 18.40% | −0.166 | 0.007 | 0.02 |
| Ischemic Heart Disease | 1,441 | 2,089 | 12.30% | 10.60% | −0.169 | 0.001 | 0.02 |
| Other heart disease | 1,588 | 2,543 | 12.80% | 11.90% | −0.056 | 0.29 | 0.15 |
| Lung Disease | 1,589 | 2,624 | 14.30% | 14.20% | −0.051 | 0.33 | 0.04 |
| COPDd | 1,078 | 1,431 | 9.40% | 6.90% | −0.181 | <0.001 | 0.003 |
| Active Asthma | 851 | 1,617 | 8.30% | 10.10% | 0.063 | 0.14 | 0.46 |
| Kidney Disease | 319 | 940 | 4.20% | 5.00% | 0.066 | 0.03 | 0.21 |
| Liver Disease | 248 | 625 | 3.10% | 4.40% | 0.087 | 0.001 | 0.01 |
| Hepatitis | 474 | 622 | 5.90% | 4.50% | −0.062 | 0.04 | 0.98 |
| Arthritis | 4,323 | 6,865 | 34.60% | 33.10% | 0.026 | 0.72 | 0.45 |
| Morbid Obesity | 716 | 1,429 | 5.90% | 9.50% | 0.209 | <0.001 | 0.50 |
Prevalence was adjusted for age, sex, and race/ethnicity, using general linear regressions.
Average annual prevalence change and p-trend were estimated with survey year included in the model as a continuous variable adjusting age, sex, and race/ethnicity using general linear regressions among cancer survivors.
P value for the interaction term between cancer status (yes/no) and year of survey.
COPD stands for chronic obstructive pulmonary disease.
Among participants without a cancer history, we observed similar trends in adjusted prevalence of most chronic conditions (Supplement Table S-2, Figure 2). The decreasing trend in prevalence of ischemic heart disease (p-interaction=0.02) and COPD (p-interaction=0.003) and the increasing trend of liver disease (p-interaction=0.01) were more pronounced among cancer survivors than in individuals without a cancer history (Table 2, Figure 2).
Figure 2.

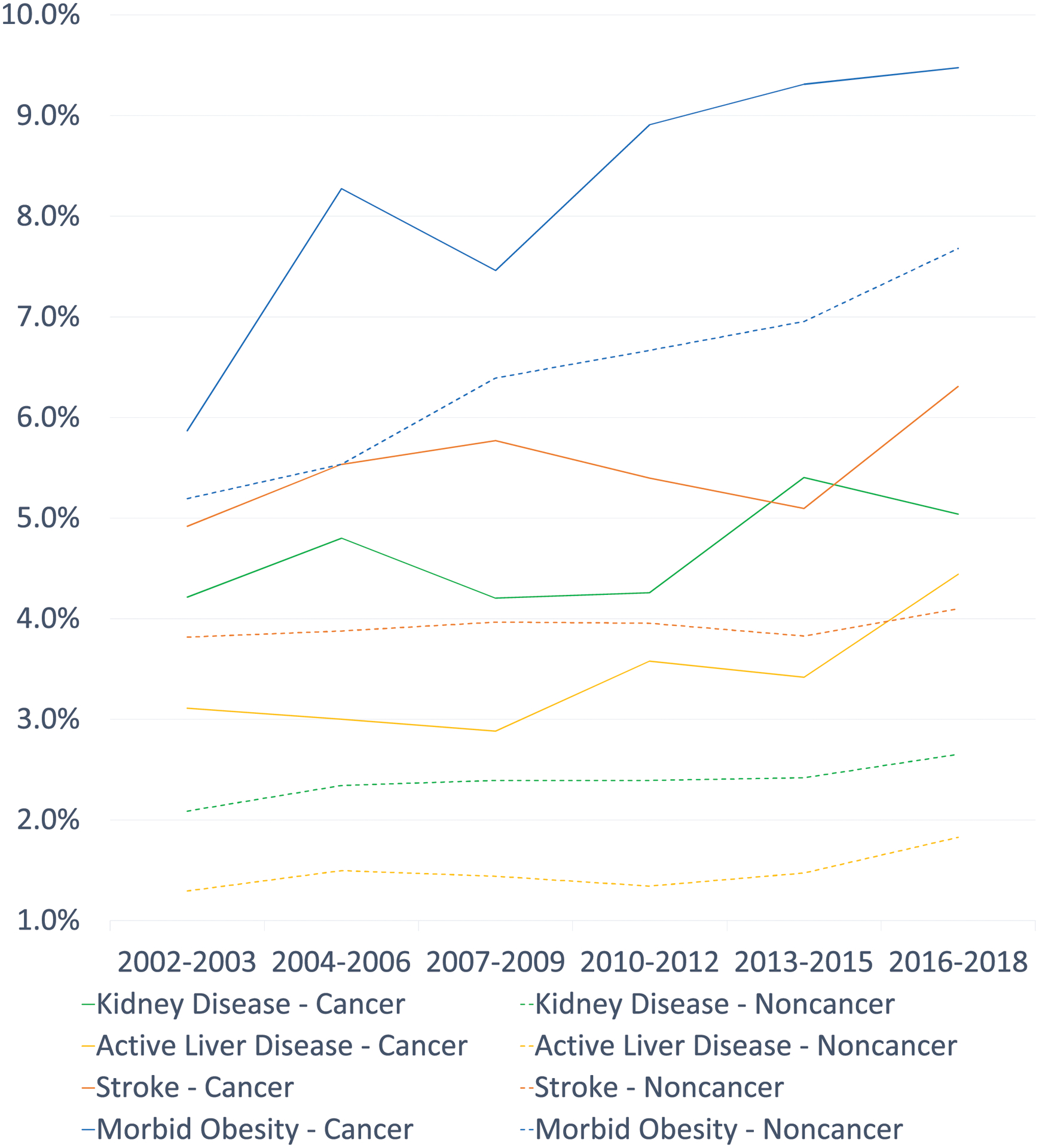
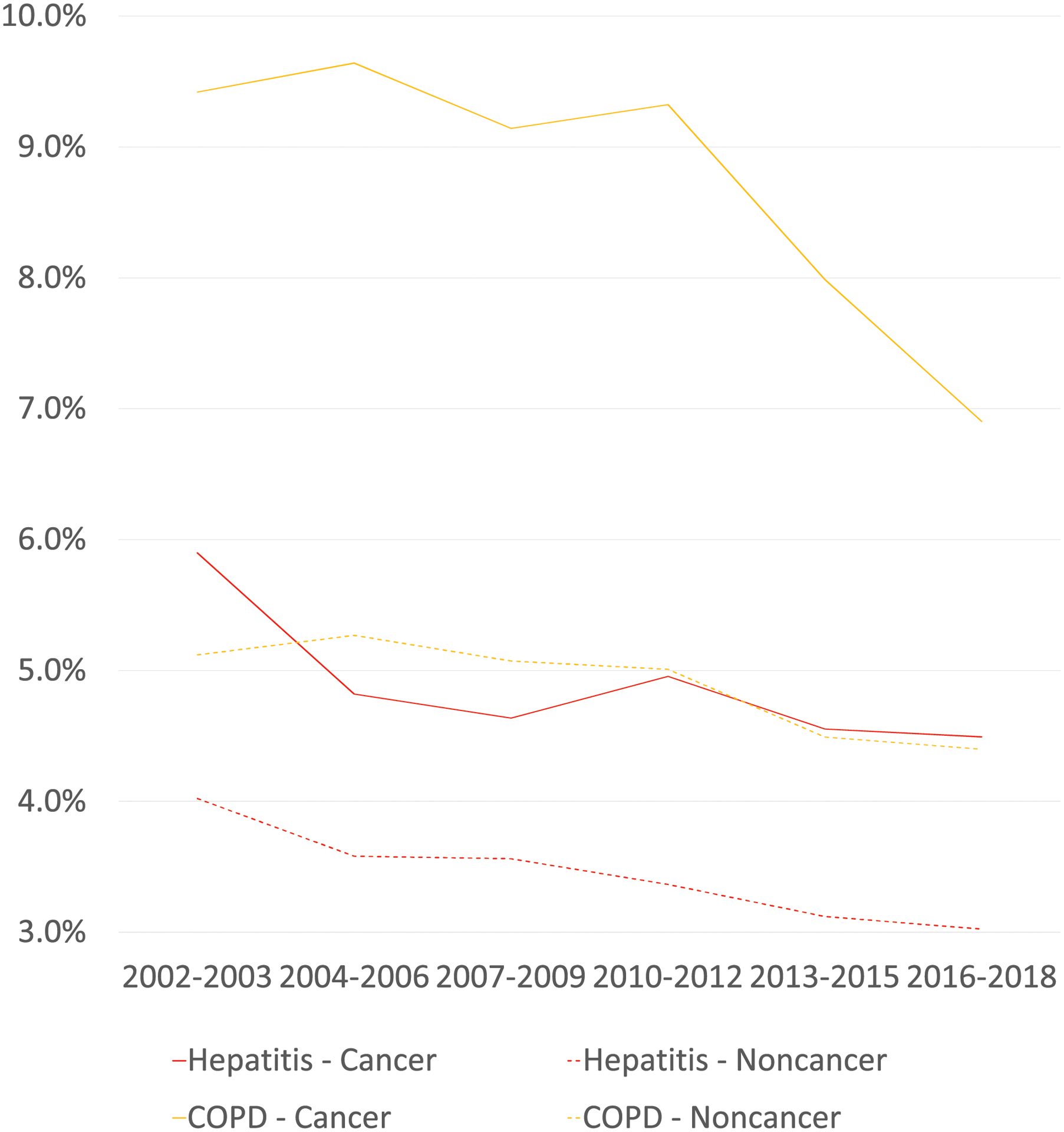
Age-sex-race/ethnicity-adjusted prevalence of chronic conditions among cancer survivors and individuals without a cancer history in the US a
a Data estimates were aggregated at 6 study periods
COPD stands for chronic obstructive pulmonary disease
The estimated population sizes of cancer survivors with chronic conditions increased by 50%−200% for almost every condition except for ischemic heart disease and COPD, which both increased by about 30% and stabilized around 2.2 million and 1.4 million respectively since 2010 (Figure 3).
Figure 3.
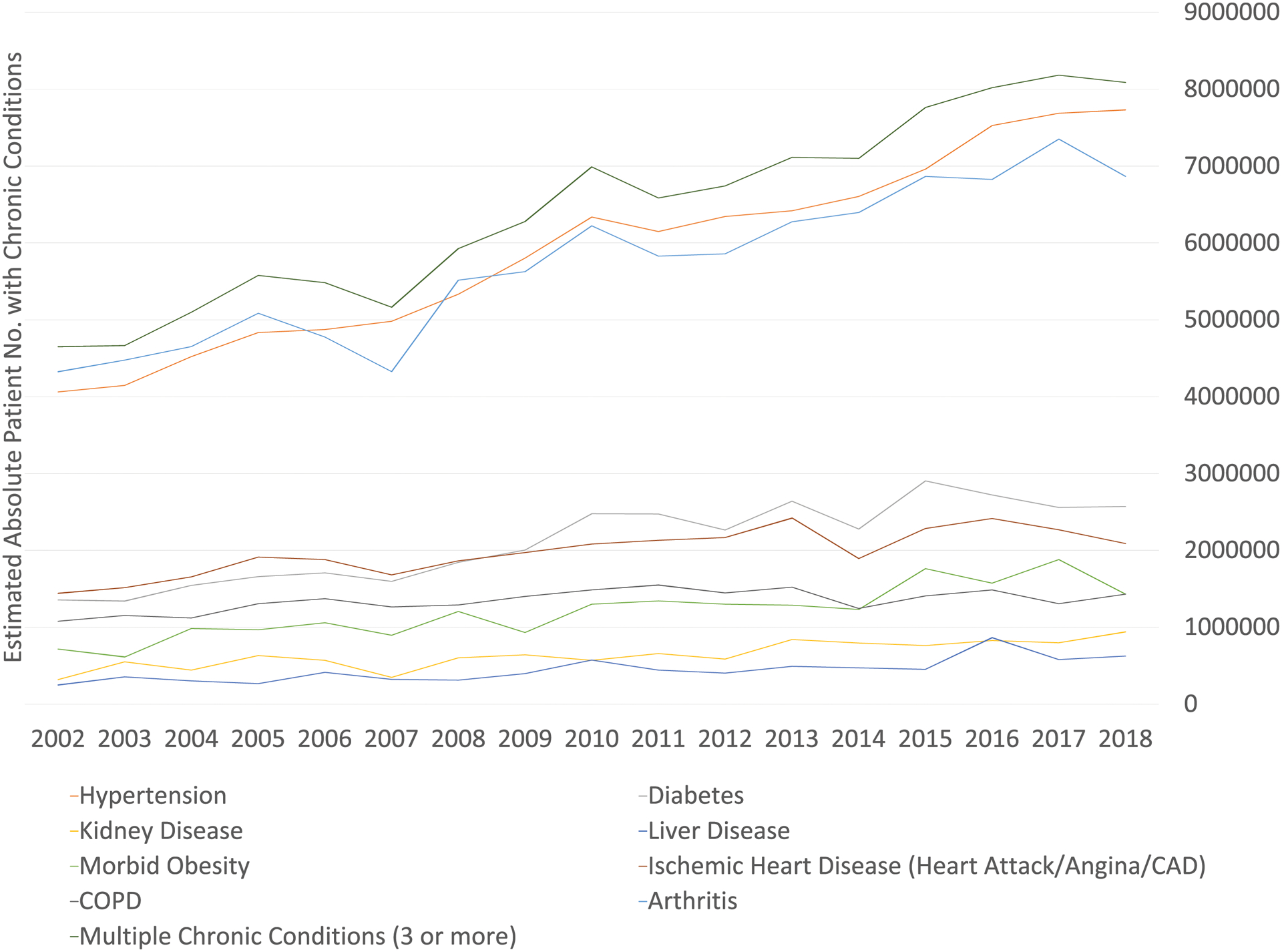
Estimated Population Sizes of Cancer Survivors with Chronic Conditions in the US a
a The absolute population with chronic conditions was estimated using National Health Interview Survey complex survey design and weights.
COPD stands for chronic obstructive pulmonary disease
Trends in Multiple Chronic Comorbidities
Cancer survivors with MCC increased significantly from 4.7 million in 2002 to 8.1 million in 2018 (Table 3, Figure 3). After adjusting for age, sex, and race/ethnicity, the prevalence of MCC increased over the study period (43.7% to 46.6%, p=0.02). The increases were most prominent among survivors aged 18–44 years old (18.4% to 28.4%, p<0.001), male survivors (44.4% to 47.9%, p=0.006), and non-Hispanic Black (56.8% to 60.4%, p=0.004) survivors. Although not statistically significant, an increasing MCC trend was also observed for most subgroups of cancer survivors such as non-Hispanic Whites and those with public insurance (Table 3).
Table 3.
Trends in Multiple Chronic Conditions (MCC, 3 or more) From 2002 to 2018 in US Adults Cancer Survivors, by Sociodemographic Factors
| Population size (2012), 1,000s | Population size (2018), 1,000s | Prevalencea (2002–2003) | Prevalencea (2016–2018) | Average annual prevalence changeb | p-Trendb | p for interactionc | |
|---|---|---|---|---|---|---|---|
| All | 4,651 | 8,089 | 43.7% | 46.6% | 0.170 | 0.02 | 0.17 |
| Age | |||||||
| 18 to 44 | 302 | 360 | 18.3% | 28.4% | 0.799 | <0.001 | <0.001 |
| 45 to 64 | 1,489 | 2,405 | 43.3% | 45.0% | 0.060 | 0.64 | 0.43 |
| 65+ | 2,860 | 5,324 | 61.9% | 62.9% | 0.108 | 0.23 | 0.21 |
| Gender | |||||||
| Male | 1,940 | 3,308 | 44.4% | 47.9% | 0.312 | 0.006 | 0.17 |
| Female | 2,711 | 4,780 | 43.6% | 46.0% | 0.080 | 0.39 | 0.54 |
| Race | |||||||
| Non-Hispanic White | 4,048 | 6,386 | 43.1% | 45.6% | 0.135 | 0.08 | 0.58 |
| Hispanic | 183 | 473 | 39.8% | 45.9% | 0.354 | 0.17 | 0.30 |
| Non-Hispanic Black | 349 | 909 | 56.8% | 60.4% | 0.671 | 0.004 | 0.02 |
| Non-Hispanic Other | 71 | 321 | 38.3% | 40.1% | −0.376 | 0.38 | 0.46 |
| Insurance | |||||||
| Private Insurance | 3,047 | 4,802 | 41.4% | 42.2% | 0.028 | 0.75 | 0.88 |
| Public Insuranced | 1,413 | 3,184 | 52.6% | 56.0% | 0.183 | 0.12 | 0.56 |
| Uninsured | 192 | 103 | 34.6% | 40.1% | 0.459 | 0.13 | 0.17 |
Prevalence was adjusted for age, sex, and race/ethnicity, using general linear regressions.
Average annual prevalence change and p-trend were estimated with survey year included in the model as a continuous variable adjusting age, sex, and race/ethnicity using general linear regressions among cancer survivors.
P value for the interaction term between cancer status (yes/no) and year.
Public insurance included self-reported Medicaid, Medicare, military health care/Veterans Affairs insurance, Medi-Gap, and Indian Health Service insurance.
Compared with individuals without a cancer history, the prevalence of MCC grew more rapidly among younger survivors (cancer:18.4% to 28.4% vs non-cancer: 1.9% to 2.9%, p-interaction =0.0004) and non-Hispanic Black survivors (from 56.8% to 60.4% among cancer survivors vs. from 19.7% to 21.8% among non-cancer individuals, p-interaction=0.02) (Table 3, Figure 4). MCC prevalence increased at a similar rate in other subgroups of cancer survivors and corresponding groups of adults without a cancer history (Table 3, Supplement Table S-3).
Figure 4.

Age-sex-race/ethnicity-adjusted prevalence of multiple chronic conditions (3 or more) by age group among cancer survivors and individuals without a cancer history in the US a
a Data estimates were aggregated at 6 study periods
The estimated population size of cancer survivors with MCC increased by 72% from 4.7 million in 2002 to 8.1 million in 2018. Survivors who were 65 years and older, female, non-Hispanic White, or public insured experienced the largest population increase in MCC (Figure 5).
Figure 5.

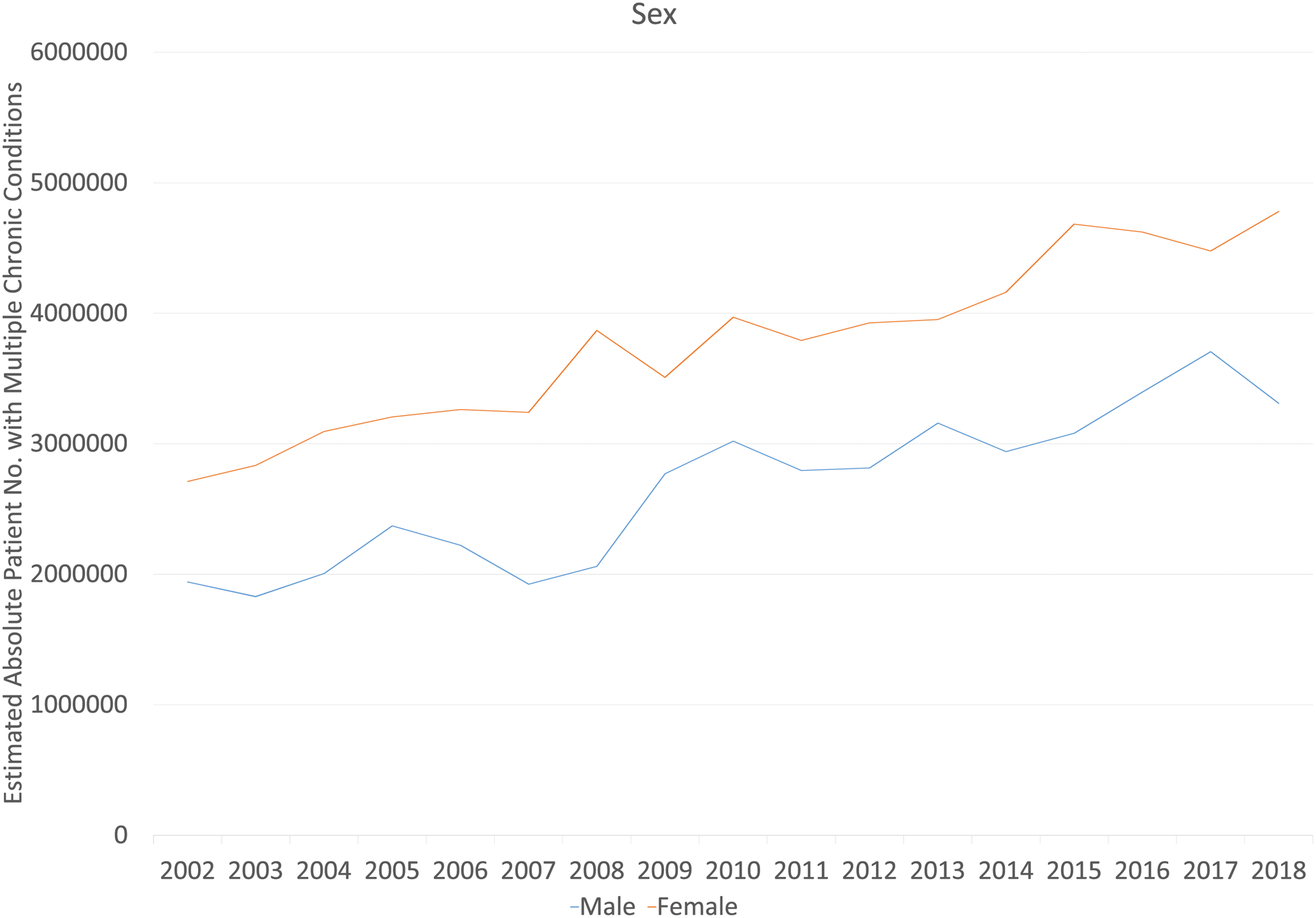
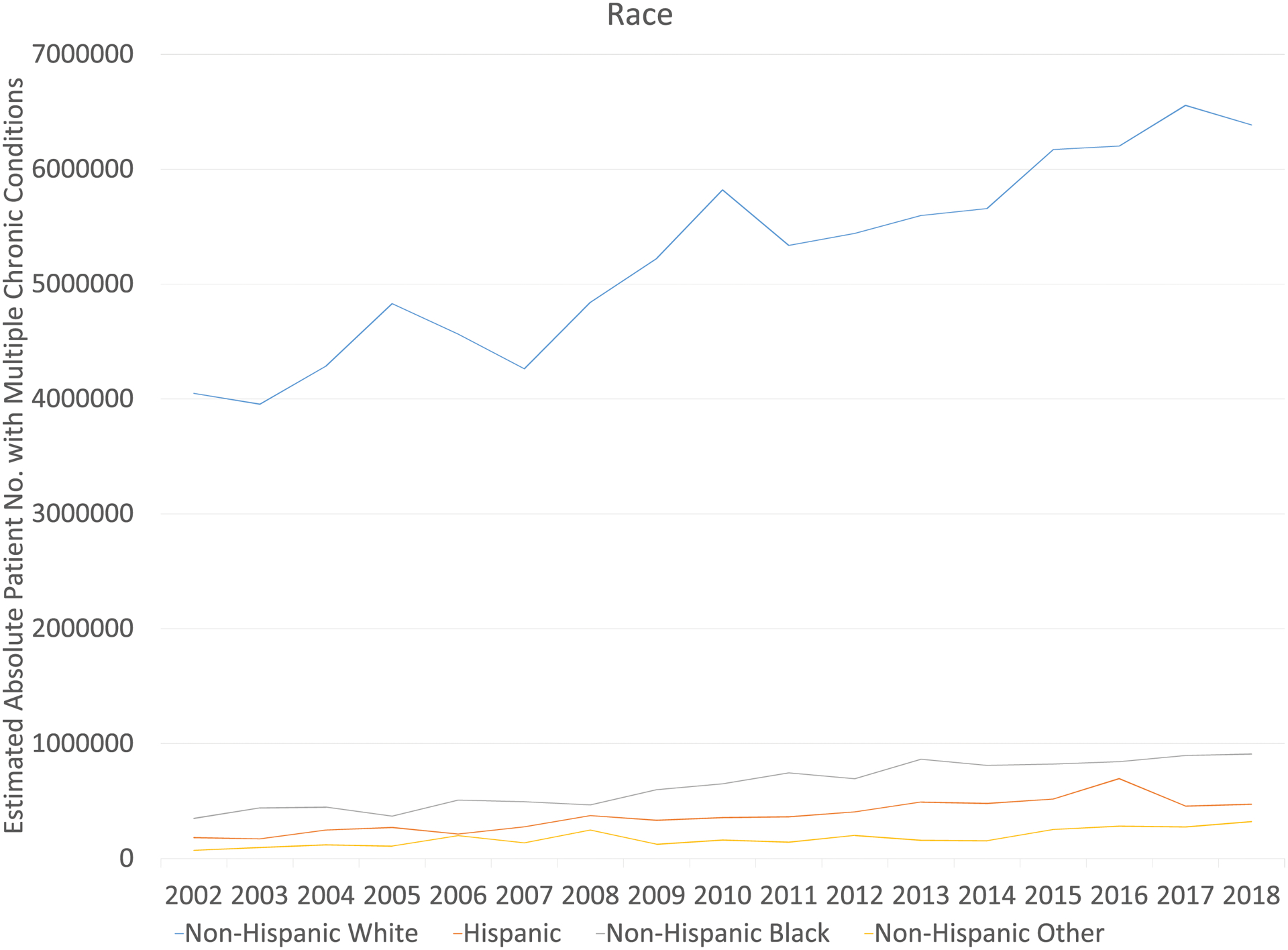

Estimated Population Sizes of Cancer Survivors with Multiple Chronic Conditions (3 or more) by Demographic and Clinical Factors in the USa
a The weighted population with multiple chronic conditions was estimated based on National Health Interview Survey complex survey design and weights.
Discussion
Our analyses demonstrate that in adult cancer survivors in the US from 2002–2018, the prevalence of cardiac disease, COPD, and hepatitis decreased while prevalence of hypertension, diabetes, kidney disease, liver disease, and morbid obesity as well as MCC increased. Our study also found that the prevalence of MCC in young (age 18–44) and non-Hispanic Black survivors grew much more rapidly than their peers without a cancer history, whereas the prevalence of MCC in other subgroups increased at similar rates in cancer survivors and individuals without a cancer history. Given the growing number of cancer survivors and increasing prevalence of MCC, the estimated population size of cancer survivors with MCC increased from 4.7 million in 2002 to 8.1 million in 2018. To our knowledge, this study provides the first national estimation of the changes in prevalence and burden of chronic conditions and MCC among cancer survivors in the past two decades.
The increasing prevalence of comorbidities among cancer survivors may be a result of the aging population, improved cancer survival, cancer treatment toxicities, and shared risk factors such as smoking, physical inactivity, and obesity.2,3,20,21 A concerning trend is the increasing prevalence of hypertension and diabetes among cancer survivors, which may be related to the rapidly increasing obesity rate in the US.22 We also found that the general population experienced a similar increase in prevalence of hypertension, diabetes, and obesity, consistent with previous studies.23,24 Thus, irrespective of a patient’s cancer history, our study demonstrates a significant growth of metabolic comorbidities in adults in the US from 2002–2018, highlighting the importance of promoting healthy lifestyles at the population level. Barriers to these broad lifestyle changes include aging, financial hardship, and limited access to health services. Although the general population may experience some of these obstacles, cancer patients in particular are susceptible given their complicated medical histories and treatment-related costs.2,5,25,26
The prevalence of kidney and liver diseases also increased over the study period, which may be a result of 1) improved patient survival, especially for those with kidney and liver diseases at cancer diagnosis; 2) treatment-related kidney and liver toxicities from curative chemotherapy or chemo-radiation therapy (e.g., platinum-based treatments); 3) the increasing rate of morbid obesity, which is a shared risk factor for both non-alcoholic steatohepatitis and chronic kidney diseases.27–29
The decreasing prevalence of both ischemic heart disease is likely a result of multiple factors. Prior studies showed that cancer survivors had a high mortality from cardiovascular disease.30,31 However, recent advances in cancer treatment, such as more precise chemo dosing regimens, improvements in radiation therapy that permit minimal cardiac exposure, and reduction in off-target effects through the wider use of targeted treatments, may be mitigating some of the negative long-term cardiovascular outcomes in cancer patients, especially in those at elevated cardiac risk.31–35 High-risk patients also benefited from advances of cardio-oncology including better cardiac risk prediction models, more sensitive biomarkers and imaging studies, and early intervention.36 Cardio-oncology guidelines now recommend clinicians to omit or replace cardiotoxic therapy (e.g., anthracycline) to prevent cardiac dysfunctions for high-risk patients such as the seniors and those with preexisting cardiac diseases.36 In addition, non-cancer related factors, such as decline in tobacco use, might also contribute to the downtrend in prevalence of ischemic heart disease and COPD in both adult cancer survivors and individuals without a history of cancer, because the decreasing trends were relatively similar for both groups.35
Our findings showed that in most subgroups the MCC prevalence among cancer survivors grew at similar rates as individuals without a cancer history. We posit that the overall increase is less likely to be cancer-treatment related but rather is linked to the increasing trend of pre-existing comorbidities and poorly controlled lifestyle factors (e.g. diet and physical activity) among the general population.24,37 To reduce the incidence of long-term comorbid illnesses and alleviate the burden of MCC, it may be necessary to consider more aggressive preventive interventions on unfavorable lifestyle factors before and after cancer diagnosis. Similar interventions would also likely benefit the general population.
One additional important observation is that young adult survivors did demonstrate a significantly rapidly increasing rate of MCC prevalence compared to their non-cancer peers. In addition to uncontrolled risks factors such as physical inactivity, obesity, and persistent tobacco use38–40, this finding may also be explained by the fact that survival and life expectancy of adolescent and young adult cancer patients had improved substantially in the past decades and they had an increasing risk of developing chronic conditions given the long-term and delayed treatment effects during their survivorship in young adulthood.41–43 It highlights the urgent need for age-appropriate survivorship guidelines, patient education, and active surveillance for young cancer survivors.
Prior malignancy and comorbid illnesses can alter physicians’ treatment decisions and clinical outcomes for both cancers and non-cancer diseases in cancer survivors.11 However, most current guidelines of cancer treatment still consider the active cancer as a single illness to manage but lack consideration for the complex interrelations between cancer and other chronic diseases.44 As a result, patients are often not able to receive optimal treatments for their cancers and other chronic diseases.8 These comorbid diseases may also exclude these survivors from clinical trials if their original cancer recurs or they develop an additional primary malignancy. Future efforts are warranted to carefully evaluate and improve the current guidelines on cancer treatment taking into consideration the prevalent comorbid conditions.
The rising MCC among cancer survivors, especially young adult survivors, poses a challenge to their primary care physicians (PCPs). As cancer prognoses improve, PCPs are playing an increasingly pivotal role in survivorship care coordination, health education, and identification and co-management of cancer treatment-related complications.20 PCPs are also critical to assisting cancer survivors improve their health-related quality of life, and alleviating the long-term psychosocial distress from cancers or treatments.45 Multidisciplinary care is frequently warranted for cancer survivors given their complex health profile involving PCPs and specialists, potentially from different health networks.20 Further substantial efforts are needed to support PCPs to transform the care model for cancer survivors, especially in underserved communities.46
This study has several strengths. First, NHIS is nationally representative, making our results generalizable to the entire US adult cancer survivor population. The US CDC has used NHIS to estimate the burden of chronic conditions and MCC in the general public.47 Second, this study analyzes the national data since 2002 to reveal the temporal trends and patterns of chronic conditions and MCC among adult cancer survivors and their peers without a cancer history. With a large number of cancer survivors and consistent ascertainment of cancer history and chronic conditions across the survey years, we were able to estimate the trends in MCC burden by various sociodemographic factors.
There were several limitations to our study. First, the NHIS collected only 10 chronic conditions, which left other conditions (e.g., dementia, vascular disease, HIV infection) unaccounted for, leading to a potential underestimation of MCC. Second, chronic conditions were self-reported in our study, which could potentially lead to underreporting of medical conditions. However, previous studies have endorsed the validity of self-reported chronic conditions in comparison with conditions based on chart review in cancer patients.48 Third, we were not able to consider cancer treatment and sequence of cancer and comorbidity diagnoses in our analysis as this information was unavailable. Fourth, our study is subject to ascertainment bias given that certain chronic conditions may be more frequently screened for in the cancer survivor population. Fifth, comorbidity burden may be underestimated due to sampling bias because patients with MCC may be less likely to participate in the NHIS interviews. In addition, the NHIS does not interview those in long-term care institutions (nursing homes, hospitals, etc.), who likely have more chronic conditions than the general population. Our study did not analyze the trends in Asians/Pacific Islanders due to limited sample size. Several conditions (e.g., insulin-dependent diabetes, active asthma, arthritis) showed a slightly increasing trend in individuals without a cancer history but were unchanged among cancer survivors. This may be due to insufficient power for cancer survivors and warrants future monitoring. Lastly, since the survey captures mostly long-term survivors of cancers with relatively better prognosis, we could not estimate chronic condition burden among shorter-term survivors and cancer patients under active treatment.
Conclusion
Our findings suggest that comorbid illnesses and MCC are an emerging public health burden for cancer survivors, particularly younger cancer survivors. Our study emphasizes the importance of collaboration between oncologists and primary care physicians, and the significant need for effective risk factor interventions in the rapidly growing adult cancer survivor population in the US.
Supplementary Material
Author disclosures:
Xuesong Han received research support from AstraZeneca for research outside of the current study. Dr. Han is employed by the American Cancer Society, Inc., a not-for-profit public health organization that receives support from the public through fundraising and direct contributions. The Society also receives a small portion of support from corporations and industry to support its mission programs and services.
The role of the funder:
This work was supported by Roswell Park Comprehensive Cancer Center and National Cancer Institute (NCI) grant P30CA016056.
Footnotes
Conflicts of Interest: None
Disclaimers: None.
Data Availability
The data underlying this article are available in National Center for Health Statistics, National Health Interview Survey, at https://www.cdc.gov/nchs/nhis/index.htm
Reference
- 1.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 2.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. New England Journal of Medicine. 2006;355(15):1572–1582. [DOI] [PubMed] [Google Scholar]
- 5.Yabroff KR, Dowling EC, Guy GP Jr., et al. Financial Hardship Associated With Cancer in the United States: Findings From a Population-Based Sample of Adult Cancer Survivors. J Clin Oncol. 2016;34(3):259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial Hardships Experienced by Cancer Survivors: A Systematic Review. J Natl Cancer Inst. 2017;109(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman KE, McCarthy EP, Recklitis CJ, Ng AK. Psychological distress in long-term survivors of adult-onset cancer: results from a national survey. Arch Intern Med. 2009;169(14):1274–1281. [DOI] [PubMed] [Google Scholar]
- 8.Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, Norgaard M. The impact of comorbidity on cancer survival: a review. Clin Epidemiol. 2013;5(Suppl 1):3–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taplin SH, Barlow W, Urban N, et al. Stage, age, comorbidity, and direct costs of colon, prostate, and breast cancer care. J Natl Cancer Inst. 1995;87(6):417–426. [DOI] [PubMed] [Google Scholar]
- 10.Guy GP Jr., Yabroff KR, Ekwueme DU, Rim SH, Li R, Richardson LC. Economic Burden of Chronic Conditions Among Survivors of Cancer in the United States. J Clin Oncol. 2017;35(18):2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66(4):337–350. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro CL, Jacobsen PB, Henderson T, et al. ReCAP: ASCO Core Curriculum for Cancer Survivorship Education. J Oncol Pract. 2016;12(2):145, e108–117. [DOI] [PubMed] [Google Scholar]
- 13.Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults—United States, 2018. Morbidity and Mortality Weekly Report. 2019;68(45):1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA. 2016;315(21):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics. National Health Interview Survey, 2000–2017. Public-use data file and documentation. In: National Center for Health Statistics; Hyattsville, MD; 2018. [Google Scholar]
- 16.Parsons VL, Moriarity C, Jonas K, Moore TF, Davis KE, Tompkins L. Design and estimation for the national health interview survey, 2006–2015. Vital Health Stat 2. 2014(165):1–53. [PubMed] [Google Scholar]
- 17.Park ER, Peppercorn J, El-Jawahri A. Shades of Survivorship. Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw. 2018;16(10):1163–1165. [DOI] [PubMed] [Google Scholar]
- 18.Berry LL, Davis SW, Godfrey Flynn A, Landercasper J, Deming KA. Is it time to reconsider the term “cancer survivor”? Journal of psychosocial oncology. 2019;37(4):413–426. [DOI] [PubMed] [Google Scholar]
- 19.National Institutes of Health. National Heart, Lung and Blood Institute, North American Association for the Study of Obesity. The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. 2000.
- 20.Leach CR, Weaver KE, Aziz NM, et al. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv. 2015;9(2):239–251. [DOI] [PubMed] [Google Scholar]
- 21.Kjaer TK, Andersen EAW, Winther JF, et al. Long-term Somatic Disease Risk in Adult Danish Cancer Survivors. JAMA Oncol. 2019;5(4):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenlee H, Shi Z, Sardo Molmenti CL, Rundle A, Tsai WY. Trends in Obesity Prevalence in Adults With a History of Cancer: Results From the US National Health Interview Survey, 1997 to 2014. J Clin Oncol. 2016;34(26):3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu JK, Charles LE, Fekedulegn D, et al. Temporal trends in prevalence of cardiovascular disease (CVD) and CVD risk factors among U.S. older workers: NHIS 2004–2018. Ann Epidemiol. 2021;55:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buttorff C, Ruder T, Bauman M. Multiple chronic conditions in the United States. Rand; Santa Monica, CA; 2017. [Google Scholar]
- 25.Sanford NN, Lam MB, Butler SS, et al. Self-reported Reasons and Patterns of Noninsurance Among Cancer Survivors Before and After Implementation of the Affordable Care Act, 2000–2017. JAMA Oncol. 2019:e191973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yabroff KR, Lawrence WF, Clauser S, Davis WW, Brown ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96(17):1322–1330. [DOI] [PubMed] [Google Scholar]
- 27.Ix JH, Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: the roles of fetuin-A, adiponectin, and AMPK. Journal of the American Society of Nephrology. 2010;21(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LaBrecque DR, Abbas Z, Anania F, et al. World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Journal of clinical gastroenterology. 2014;48(6):467–473. [DOI] [PubMed] [Google Scholar]
- 29.Mount PF, Juncos LA. Obesity-related CKD: when kidneys get the munchies. In: Am Soc Nephrol; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. European heart journal. 2019;40(48):3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Darby SC, McGale P, Taylor CW, Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. The lancet oncology. 2005;6(8):557–565. [DOI] [PubMed] [Google Scholar]
- 32.Rich TA, Shepard RC, Mosley ST. Four decades of continuing innovation with fluorouracil: current and future approaches to fluorouracil chemoradiation therapy. Journal of Clinical Oncology. 2004;22(11):2214–2232. [DOI] [PubMed] [Google Scholar]
- 33.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors: A Review. JAMA Oncology. 2016;2(10):1346–1353. [DOI] [PubMed] [Google Scholar]
- 34.Goyal G, Silberstein PT, Armitage JO. Trends in use of radiation therapy for Hodgkin lymphoma from 2000 to 2012 on the basis of the National Cancer Data Base. Clinical Lymphoma Myeloma and Leukemia. 2016;16(1):12–17. [DOI] [PubMed] [Google Scholar]
- 35.Talluri R, Fokom Domgue J, Gritz ER, Shete S. Assessment of Trends in Cigarette Smoking Cessation After Cancer Diagnosis Among US Adults, 2000 to 2017. JAMA Network Open. 2020;3(8):e2012164–e2012164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamo CE, Bloom MW, Cardinale D, et al. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 2: Prevention, Treatment, Guidelines, and Future Directions. Circ Heart Fail. 2016;9(2):e002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health. 2005;26:421–443. [DOI] [PubMed] [Google Scholar]
- 38.Tseng TS, Lin HY, Martin MY, Chen T, Partridge EE. Disparities in smoking and cessation status among cancer survivors and non-cancer individuals: a population-based study from National Health and Nutrition Examination Survey. J Cancer Surviv. 2010;4(4):313–321. [DOI] [PubMed] [Google Scholar]
- 39.Gallaway MS, Glover-Kudon R, Momin B, et al. Smoking cessation attitudes and practices among cancer survivors - United States, 2015. J Cancer Surviv. 2019;13(1):66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein C, Colditz G. Modifiable risk factors for cancer. British journal of cancer. 2004;90(2):299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao C, Bhatia S, Xu L, et al. Chronic Comorbidities Among Survivors of Adolescent and Young Adult Cancer. Journal of Clinical Oncology. 2020;38(27):3161–3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L, Moke DJ, Tsai KY, et al. A Reappraisal of Sex-Specific Cancer Survival Trends Among Adolescents and Young Adults in the United States. J Natl Cancer Inst. 2019;111(5):509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderson C, Nichols HB. Trends in Late Mortality Among Adolescent and Young Adult Cancer Survivors. J Natl Cancer Inst. 2020;112(10):994–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimmick G, Fleming ST, Sabatino SA, et al. Comorbidity burden and guideline-concordant care for breast cancer. J Am Geriatr Soc. 2014;62(3):482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eton DT, Anderson RT, Cohn WF, et al. Risk factors for poor health-related quality of life in cancer survivors with multiple chronic conditions: exploring the role of treatment burden as a mediator. Patient Relat Outcome Meas. 2019;10:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chaput G, Med CP, Sussman J. Integrating primary care providers through the seasons of survivorship. Curr Oncol. 2019;26(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ye F, Moon DH, Carpenter WR, et al. Comparison of patient report and medical records of comorbidities: results from a population-based cohort of patients with prostate cancer. JAMA oncology. 2017;3(8):1035–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in National Center for Health Statistics, National Health Interview Survey, at https://www.cdc.gov/nchs/nhis/index.htm


