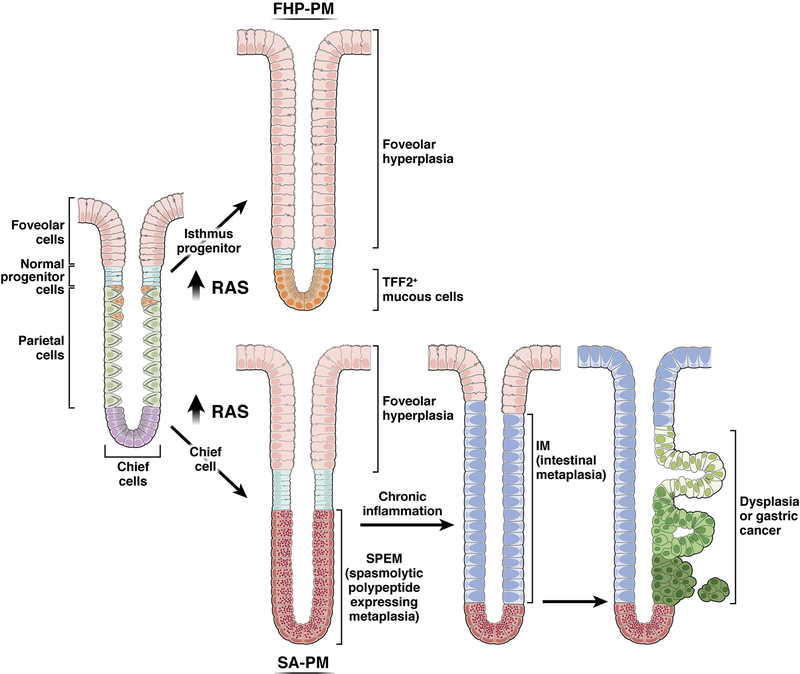
Figure 3: The divergent effects of Ras activation in isthmal progenitor cells and chief cells establish two types of pyloric metaplasia.
Increases in Ras activation in isthmal progenitor cells in transgenic mice and in the setting of Ménétrier’s disease leads to massive foveolar hyperplasia (foveolar hyperplasia predominant pyloric metaplasia, FHP-PM) with preferential differentiation of foveolar cells, and to a lesser extent mucous neck cells, over parietal cells. In contrast, activation of Ras in chief cells leads initially to the development of SPEM from reprogramming of chief cells (SPEM-associated pyloric metaplasia, SA-PM). Continuously active Ras expression can lead to intestinal metaplasia and dysplasia, establishing the full range of pre-neoplastic lineages associated with the development of intestinal type gastric cancer. Note: the intestinal metaplasia depicted here would be of the “incomplete type.” The figure is not meant to show definitively which exact cells are the ones fueling the expansion of dysplastic/neoplastic cells, but evidence does point to lineages at the interface of intestinal metaplasia arising from pyloric metaplasia/SPEM as the figure depicts.