Abstract
Background:
Children whose parents have type 2 diabetes (T2D) are at high-risk for developing T2D. In youth, negative affect has been shown to predict insulin resistance (IR), and disinhibited-eating behaviors have been linked to IR. It is unknown if youth with a parent with T2D (P-T2D) report greater psychological and behavioral symptoms than those without a P-T2D.
Objective:
To compare youth with and without a P-T2D on symptoms of negative affect and disinhibited-eating.
Methods:
Nine-hundred-thirty-two youth (13.3±2.6 y; BMIz 1.06±1.06; 67.8% female; 53.6% People of Color; 10.7% with a P-T2D) completed questionnaires of anxiety and depressive symptoms, eating in the absence of hunger, and emotional-eating. Loss-of-control (LOC)-eating was assessed by interview. In two separate sub-samples, energy intake was explored using laboratory test meals simulating eating in the absence of hunger and LOC-eating, respectively. Analyses were adjusted for age, sex, race/ethnicity. In follow-up analyses, fat mass (kg) and height, and IR were included as covariates, respectively.
Results:
Adjusting for all covariates including adiposity and IR, compared to youth without a P-T2D, youth with a P-T2D reported more anxiety and depression symptoms, greater eating in the absence of hunger, and emotional-eating (ps<.05). No significant differences were found for LOC-eating, or in exploratory analyses of energy intake for either test meal (ps>.16).
Conclusions:
Self-reported negative affect and disinhibited-eating may be higher among youth with P-T2D compared to those without P-T2D. Prospective studies should examine, among those with a P-T2D, what role such symptoms may play for their subsequent risk for T2D.
Keywords: Parents, type 2 diabetes, negative affect, disinhibited eating, insulin resistance
Graphical Abstract
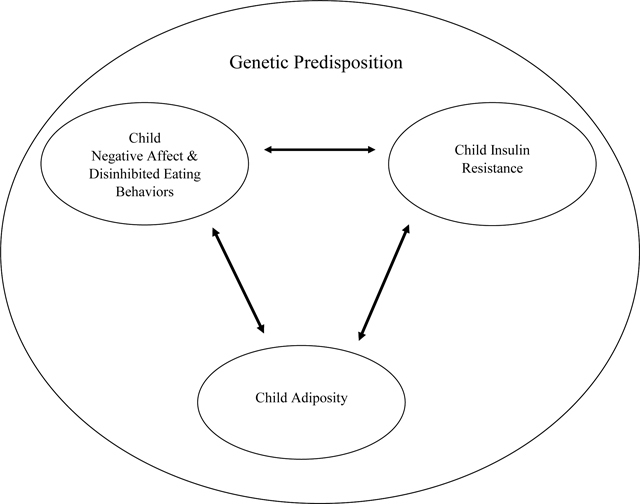
Psychological symptoms, such as negative affect and disinhibited eating, may be higher among youth genetically predisposed to type 2 diabetes based on parent diabetes status, above and beyond the contribution of known risk factors, such as obesity and insulin resistance.
Introduction
Paralleling the increased prevalence of obesity in U.S. youth,1 a higher incidence of pediatric type 2 diabetes (T2D) has also been observed.2 T2D places youth at high risk for multiple disorders, including cardiovascular and kidney disease.3 Thus, it is important to understand early risk factors that may increase the likelihood of T2D development in youth. Among predictors of T2D, inherited factors play robust roles concurrent with environmental factors, such as poor diet and physical inactivity.3, 4 Offspring are at a 3.5-fold increased risk of developing T2D when just one parent has T2D.5, 6 When both parents have T2D, this risk increases 6-fold.5, 6 Given the strong link between parental body mass index (BMI, kg/m2) and offspring BMI,7, 8 it is not surprising that child BMI is also an important predictor of insulin resistance (IR), the primary physiological precursor to T2D.9–11
In adults, psychological factors, including anxiety and depression, have been cross-sectionally and prospectively associated with the development of IR and T2D, especially among women.12–15 A meta-analysis found that adults with depression or elevated depressive symptoms have a 37% increased risk of developing T2D;16 conversely, meta-analytic findings also indicate that adults with T2D have a 24% increased risk of developing depression.17 Further, disordered eating behaviors, such as binge-eating disorder, may be prospective indicators for development of T2D.18–20 Some of these findings have been replicated in pediatric samples.21–26 Cross-sectional findings suggests that anxiety has been less consistently associated with IR in youth.21, 25 Accounting for adiposity, depressive symptoms have been associated with25 and predictive of24 worsening IR. In youth, both binge- and loss-of-control (LOC)-eating have been linked to IR or T2D.21–23, 26 These childhood symptoms may confer additional risk when a parent has T2D (Figure 1 shows Conceptual Model).
Figure 1.
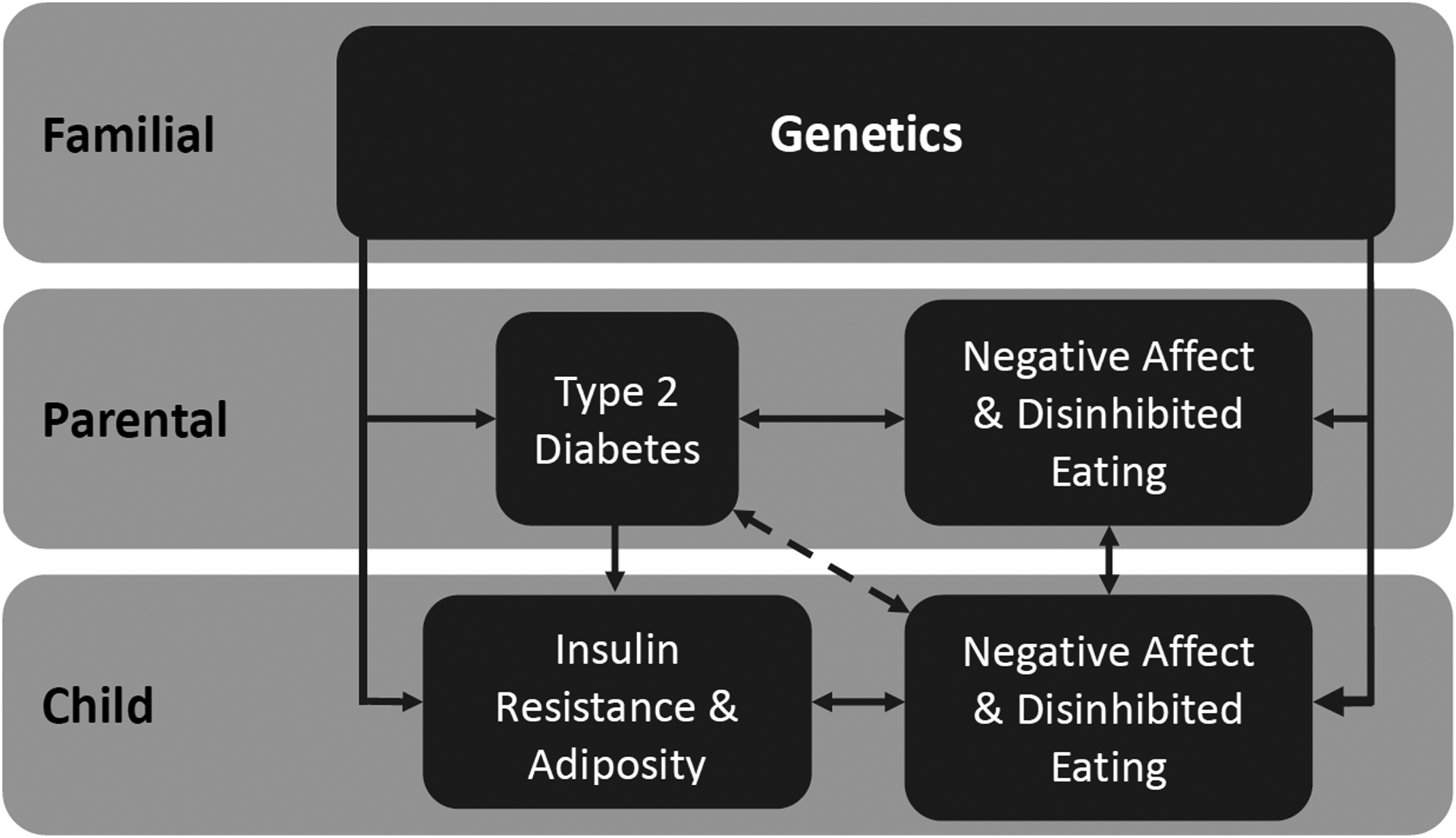
Conceptual Model. Children of parents with type 2 diabetes may have negative affect and disinhibited eating symptoms, above and beyond additional familial risk for type 2 diabetes.
To gain an initial understanding of whether psychological symptoms may be associated with genetic predisposition for T2D, we examined negative affect and disinhibited eating (an overall construct that include eating in the absence of hunger, EAH, emotional-eating, and LOC-eating) behaviors in youth with and without a parent with T2D (P-T2D). We hypothesized that compared to children for whom neither biological parent has T2D, children having at least one P-T2D would be more likely to have higher anxiety, depressive symptoms, and disinhibited eating behaviors. We also explored whether, within the context of a laboratory test meal, children having a P-T2D consumed more energy.
Methods
Participants
A convenience sample from seven protocols involving youths (ages 8–18 years), studied between June 1996 and November 2020, was assembled. Three studies involved interventions (ClinicalTrials.gov IDs: NCT00263536; NCT00680979; NCT01425905) and four were non-treatment (observational) protocols (ClinicalTrials.gov IDs: NCT00001522; NCT00320177; NCT00631644; NCT02390765). All protocols were approved by the Institutional Review Board of the National Institutes of Health. For all studies, youth were recruited through physician referral, flyers (directed to parents) posted in public facilities, newspaper advertisements, and mailings to families in the Washington, D.C., metropolitan area. Non-intervention studies examined psychological and physiological factors that promote obesity. Intervention studies focused on reducing psychological factors that promote excess weight gain or IR, with the aim of preventing adult obesity or worsening IR and T2D. For both the non-intervention and intervention studies, all participants were financially compensated for their time. Protocol-specific eligibility requirements included girls with LOC-eating (ClinicalTrials.gov ID: NCT00680979), being at risk for excess weight gain or with overweight/obesity (ClinicalTrials.gov ID: NCT00263536), girls with overweight, elevated depressive symptoms (but not reaching the major depressive disorder threshold), and a family history of T2D based on first- and second-degree relatives (ClinicalTrials.gov ID: NCT01425905), identification as either White or Black (ClinicalTrials.gov ID: NCT00001522), and enrichment for LOC-eating (ClinicalTrials.gov ID: NCT00320177). For the current analyses, individuals were included if they were in good general health. See Table 1. Youth were excluded based on identification of any major medical illness (including T2D), full-syndrome psychiatric disorder (other than binge-eating disorder), or prescription of any medications or therapy that may impact weight. This study is the first to explore the relationship between parental T2D and obesity-related outcomes using data from these trials.
Table 1.
Intervention and Non-Intervention Protocols indicated by Clinical Trials Identification Number and Primary Inclusion Criteria for Studies Comprising the Combined Sample
| Intervention Protocols | n | Inclusion Criteria |
|---|---|---|
| NCT00263536 | 57 |
|
| NCT00680979 | 116 |
|
| NCT01425905 a | 107 |
|
| Non-Intervention Protocols | ||
| NCT00001522 | 25 |
|
| NCT00320177 b | 228 |
|
| NCT00631644 | 165 |
|
| NCT02390765 | 234 |
|
Protocol used for the exploratory intake analyses using the eating in the absence of hunger test meal
Protocol used for the exploratory intake analyses using the loss-of-control eating test meal
Procedures
Written informed consent from parents/guardians and assent from children/adolescents were obtained prior to study participation. All children and their parent/guardian were seen at the Hatfield Clinical Research Center, National Institutes of Health (NIH), Bethesda, Maryland, USA. For the intervention protocols, data were collected at baseline, prior to treatment.
Measures
Parent-Reports:
Child’s race and ethnicity and biological parents’ T2D status was reported during their child’s health history with a nurse practitioner or an endocrinologist.
Body measurements.
BMI was calculated from height and fasting weight. Height was measured to the nearest millimeter with a calibrated wall stadiometer from the average of three measurements. Weight was measured to the nearest 0.1 kg with a calibrated digital scale. BMI was adjusted based on age and sex, and standardized BMI (BMIz) was determined according to CDC growth charts.27
Body composition was measured using dual-energy X-ray absorptiometry (DXA; Hologic QDR2000 or QDR4500, Bedford, MA, USA; or iDXA system; GE Healthcare, Madison, WI, USA) or air displacement plethysmography (Bod Pod; Life Measurement Inc., Concord, CA, USA). Both methods are validated measures of body composition28–30 and have been successfully combined in previous studies. e.g.31 Prior to combining DXA and Bod Pod data, adjustments were applied to improve comparability between methods, as previously described.32
Insulin resistance.
IR was determined using blood samples obtained in the morning after an overnight fast to measure serum insulin and glucose, as previously described.21 IR was estimated using the homeostasis model assessment of IR (HOMA-IR) index: (fasting insulin [mU/mL]*fasting glucose [mmol/L])/22.5.33
Negative affect:
The State-Trait Anxiety Inventory for Children trait scale34 is a 20-item self-report measure of trait anxiety. Items assessing anxiety-related symptoms are rated on a 3-point Likert scale ranging from 1= “hardly ever” to 3= “often.” The total score is the sum of all items, with higher scores indicating greater symptoms of trait anxiety. This measure has demonstrated good internal consistency and good test-retest reliability.34 In the current sample Cronbach’s α was .88. The Children’s Depression Inventory35 is a 27-item measure that assesses depressive behaviors and cognitions in the past two weeks. Items are rated on a 0 (absence of symptom) to 3 (high severity of symptom) scale. The total score is the sum of all items, with higher scores indicating greater levels of depressive symptoms. This measure has demonstrated good internal consistency and convergent and discriminant validity.36 This measure was not obtained in two protocols included for analyses (ClinicalTrials.gov IDs: NCT00680979, NCT00631644). Internal consistency in the current sample was good (Cronbach’s α = .86).
Disinhibited eating.
The Eating in the Absence of Hunger (EAH) for Children scale37 is a 14-item measure used to assess eating when not hungry or past satiation in response to external cues (e.g. “how often do you keep eating because others are still eating?”); fatigue and boredom (e.g. “how often do you keep eating because you are feeling bored”); and negative affect (e.g. “how often do you keep eating because you are feeling anxious or nervous?”). Items are rated on a 5-point Likert scale ranging from 0= “never” to 4= “always.” The average total score of all items was used, with higher scores indicating greater EAH. This measure has demonstrated adequate to good psychometric properties.37 Cronbach’s α was .90 for the current sample.
The Emotional Eating Scale for Children and Adolescents38 is a 25-item self-report measure to describe a desire to eat based on feelings of anxiety, anger, and frustration; depressive symptoms; and feeling unsettled. Total scores are averaged from all items, with a scale ranging from 0 (having no desire to eat) to 4 (having a very strong desire to eat). This measure has demonstrated good psychometric properties,38 and construct validity for the total score, with observed eating behavior.39 Internal consistency in the current sample was excellent (Cronbach’s α = .95).
The Eating Disorder Examination40 or the child version41 is a semi-structured interview used to assess disordered eating attitudes and behaviors and to generate eating disorder diagnoses according to the DSM-IV42 or DSM-5.43 The interview was used to determine presence or absence of LOC-eating episodes in the past month. The child and adult versions of the interview have been successfully combined and have shown excellent inter-rater reliability for LOC-eating presence.44
Energy intake.
For exploratory analyses, test meal data were drawn from two protocols with the greatest percentage of participants with a P-T2D (ClinicalTrials.gov IDs: NCT01425905 and NCT00320177) to assess total intake of energy (kcal) and percentages for macronutrient content (i.e., protein, fat, and carbohydrate). Meal study methodologies differed across protocols and were designed to examine two of the constructs of interest. Specifically, one was designed to induce EAH among intervention girls (ClinicalTrials.gov ID: NCT01425905), whereas the other was designed to model a LOC-eating meal among non-intervention boys and girls (ClinicalTrials.gov ID: NCT00320177). Thus, meal intake data were examined separately, and the samples did not overlap. Full descriptions of the EAH45 and LOC-eating46 meal protocols have been previously reported.
In brief, for the EAH test meal, participants were served a large food array (>10,000 kcal) varied in macronutrients (55% carbohydrate, 12% protein, 33% fat)45 at approximately 12:00 p.m. following an overnight fast. An oral glucose tolerance test was administered prior to the lunch array where participants were given Glucola at approximately 9:00 a.m. Immediately prior to eating, participants were instructed to “Please eat until you are no longer hungry.” To assess EAH, approximately sixty minutes after the start of the large meal, participants were served an array of highly palatable snack foods (>4,000 kcal) and instructed to “please taste each of the foods” to rate preferences on how much they liked or disliked foods on a rating form and then eat as much as they liked.
For the LOC-eating test meal, participants were served a large food array (~9,835 kcal) varying in macronutrients (51% carbohydrate, 12% protein, 37% fat)47 at approximately 2:30 p.m., following an overnight fast and a standardized breakfast of 288 kcal (i.e., 240 mL apple juice, 1 English muffin, 6 g butter) at 8:40 a.m. Immediately prior to eating, participants were instructed to “Let yourself go and eat as much as you want.”
All food items were measured individually in grams before and after each test meal. Energy intake and macronutrient content was calculated using a metabolic diet study management system that used the U.S. Department of Agriculture Nutrient Database for Standard Reference (Agriculture Research Service, Beltsville, MD, U.S.A.)45 for the EAH meal and for the LOC-eating meal, the U.S. Department of Agriculture Nutrient Database for Standard Reference, release 16 (Viocare Technologies Inc, Princeton, NJ, U.S.A.).46
Statistical Analysis
Analyses were conducted using IBM SPSS Statistics 25. Data from all protocols were combined and screened for normality and outliers. HOMA-IR and fat mass (kg) were natural log-transformed to improve normality. Extreme but plausible outliers (>3 SD above the mean) were windsorized (n = 24) to retain cases but minimize influence on outcomes. Arcsine square root transformations were performed on all percentage variables. All covariates were tested for multicollinearity (r > .8). No variable violated assumptions of non-multicollinearity, and thus all were retained in analyses.
To compare sociodemographic characteristics of the two groups, t-test and chi-square analyses were performed. To assess differences between children with and without a P-T2D on negative affect and disinhibited eating symptoms, four analyses of covariance (ANCOVAs) were conducted for anxiety, depressive symptoms, EAH, and emotional-eating. To determine if children with at least one P-T2D were more likely than children without a P-T2D to report LOC-eating within the past month, a binary logistic regression was performed. All analyses conducted were first adjusted for age, sex, race/ethnicity (coded as 1 = non-Hispanic White, 0 = People of Color). Analyses were repeated, including fat mass (kg) and height as additional body composition covariates. Finally, analyses were repeated a third time adding HOMA-IR as an additional covariate to determine if findings persisted over and above the contribution of T2D risk factors such as adiposity and IR. Given its relevance to these relationships, socioeconomic status was considered as a covariate, but did not significantly contribute to any models and thus was not included. Follow-up analyses were repeated including intervention-seeking status (0 = non-intervention, 1 = intervention-seeking) as an additional covariate. Analyses repeated twice more; once with only non-intervention and again with intervention-seeking only participants.
For exploratory objective energy intake and macronutrient content analyses, analyses of covariance (ANCOVAs) were employed. Dependent variables included total intake of energy (kcal) and percentage macronutrient content consumed. Analyses involving total energy intake were adjusted for age, sex (as applicable, as one sample only studied females), race/ethnicity, height, followed by inclusion of lean mass/fat free mass (kg), fat mass (%), and finally HOMA-IR. Analyses involving percentage macronutrient variables were adjusted for age, sex, race/ethnicity, and HOMA-IR.
Results
Participant characteristics
A total of 932 participants (13.3 ± 2.6 years; BMIz = 1.06 ± 1.06; 67.8% female; 53.6% People of Color) were included in analyses. Of the total sample, 21.8% (n = 203) had overweight (BMI ≥85th and <95th percentile), and 34.5% (n = 322) had obesity (BMI ≥95th percentile). One-hundred (10.7%) participants had a P-T2D; of these youth, 14 had two P-T2D. Participants with one or two P-T2D were older, more likely to be female, Black, and had higher BMIz compared to participants without a P-T2D (all ps ≤ .001). Compared to children with no P-T2D, youth with just one (versus two) P-T2D were more likely to be female, Black, have overweight/obesity, to be older, and have greater IR (ps <.002). Total sample characteristics and group differences based on P-T2D status are in Table 2. Approximately two-thirds (n = 626; 67.2%) of the total sample were included in analyses examining depressive symptoms. Compared with those who did not have depressive symptom data, those with data were significantly more likely to be younger, female, White, and have greater HOMA-IR (all ps <.01). For the exploratory analyses assessing energy intake, one-hundred adolescents, all female, and 38% with a P-T2D (n = 38), completed the EAH test meal and two-hundred youth, 49% female (n = 98) and 9% with P-T2D (n = 18) completed the LOC-eating test meal.
Table 2.
Sample Characteristics
| Total Sample | No P-T2D | One or both P-T2D | p | |
|---|---|---|---|---|
| (n=932) | (n=832) | (n=100) | ||
| Age (years)a | 13.33±2.62 | 13.23±2.65 | 14.16±2.18 | 0.001 |
| Sex (n, % Female) | 632 (67.81%) | 546 (65.63%) | 86 (86%) | <.001 |
| Race (n, %) | <.001b | |||
| White | 463 (49.7%) | 435 (52.3%) | 28 (28.0%) | |
| Black or African-American | 304 (32.6%) | 250 (30.0%) | 54 (54.0%) | |
| Asian American | 55 (5.9%) | 51 (6.1%) | 4 (4.0%) | |
| Another raceb | 11 (1.2%) | 8 (1.0%) | 3 (3.0%) | |
| Ethnicity (n, % Hispanic/Latinx) | 75 (8.0%) | 62 (7.5%) | 13 (13.0%) | 0.11 |
| Non-Hispanic White | 432 (46.4%) | 407 (48.9%) | 25 (25.0%) | <.001 |
| Median SESc | 2 | 2 | 2 | 0.526 |
| BMIz | 1.06±1.06 | .97±1.07 | 1.69±.69 | <.001 |
| % Fat massd | .33±.12 | .32±.12 | .39±.10 | <.001 |
| HOMA-IRe | 3.11±3.04 | 2.91±.2.78 | 4.77±4.35 | <.001 |
| Anxiety symptoms | 32.69±7.43 | 32.34±7.33 | 35.31±7.70 | |
| Depressive symptoms | 7.50±6.15 | 7.11±5.89 | 10.29±7.24 | |
| Clinically significant depression (n, %)f | 17, 2.0% | 10, 10.0% | 27, 2.9% | |
| Eating in the absence of hunger | 27.01±8.94 | 26.45±8.644 | 31.40±9.98 | |
| Emotional-eating | 0.81±0.71 | 0.78±0.69 | 1.08±0.76 | |
| Loss-of-control eating (n, %) | 351, 40.1% | 303, 39.0% | 48, 48.5% |
P-T2D = Parent(s) with type 2 diabetes
SES = Socioeconomic status
BMIz = Age- and sex-adjusted body mass index
HOMA-IR = Homeostasis Model Assessment of Insulin Resistance
Values presented are M ± SD, unless otherwise stated
Another race included those who categorized their race as “Other”
Socioeconomic status was measured by the Hollingshead Two Factor Index of Socioeconomic Status (1975). Scale ranges from 1 to 5 with higher scores indicating higher status
For analyses in results, variable was arcsine transformed
For analyses in results, variable was natural log transformed
Based on questionnaire screening cut-off
Negative affect
In models adjusting for age, sex, race/ethnicity, children with a P-T2D reported greater symptoms of anxiety (F(1, 733) = 5.78, p = .016, ηp2 = .01) and depression (F(1, 621) = 7.26, p = .007, ηp2 = .01) compared to children without a P-T2D. When adiposity and height were included as additional covariates, findings remained significant for greater anxiety (F(1, 731) = 3.97, p = .047, ηp2 = .01) and depressive symptoms (F(1, 619) = 4.43, p = .036, ηp2 = .01). When HOMA-IR was included as an additional covariate, findings remained consistent for anxiety (F(1, 730) = 3.89, p = .049, ηp2 = .01; Table 3; Figure 2a) and depressive symptoms (F(1, 618) = 4.52, p = .034, ηp2 = .01; Table 3; Figure 2b).
Table 3.
Association of Youth having a P-T2D with Negative Affect and Disinhibited Eating Behaviors
| Dependent Variables | Independent Variables | F | p | ηp2 |
|---|---|---|---|---|
| Anxiety | P-T2D | 3.89 | .049* | .01 |
| Age | 1.07 | .302 | <.001 | |
| Sex | 11.41 | <.001*** | .02 | |
| Race/Ethnicity | 0.10 | .751 | <.001 | |
| Fat mass (kg) | 13.90 | <.001*** | .02 | |
| Height (cm) | 6.10 | .014* | .01 | |
| HOMA-IR | 0.11 | .738 | <.001 | |
| Depressive Symptoms | P-T2D | 4.52 | .034* | .01 |
| Age | 10.10 | .002** | .02 | |
| Sex | 1.64 | .201 | .001 | |
| Race/Ethnicity | 3.45 | .064 | .01 | |
| Fat mass (kg) | 4.90 | .027* | .01 | |
| Height (cm) | 11.27 | <.001*** | .02 | |
| HOMA-IR | 9.14 | .003** | .02 | |
| EAH | P-T2D | 15.85 | <.001*** | .02 |
| Age | 22.85 | <.001*** | .03 | |
| Sex | 5.25 | .022* | .01 | |
| Race/Ethnicity | 2.61 | .107 | .001 | |
| Fat mass (kg) | 4.64 | .032* | .01 | |
| Height (cm) | 4.13 | .042* | .01 | |
| HOMA-IR | 0.48 | .489 | .001 | |
| Emotional Eating | P-T2D | 6.41 | .012* | .01 |
| Age | 7.96 | .005** | .01 | |
| Sex | 10.36 | .001** | .01 | |
| Race/Ethnicity | 0.35 | .553 | <.001 | |
| Fat mass (kg) | 3.12 | .078 | .001 | |
| Height (cm) | 2.24 | .135 | .001 | |
| HOMA-IR | 0.71 | .401 | .001 | |
| Dependent Variables | Independent Variables | β | p | OR |
| LOC-eating presence | P-T2D | −0.08 | .739 | 0.92 |
| Age | −0.07 | .112 | 0.93 | |
| Sex | 0.91 | <.001 | 2.49 | |
| Race/Ethnicity | 0.01 | .944 | 1.01 | |
| Fat mass (kg) | 1.50 | <.001*** | 4.47 | |
| Height (cm) | 0.001 | .954 | 1.00 | |
| HOMA-IR | −0.41 | <.001*** | 0.66 |
P-T2D = Parent with type 2 diabetes
HOMA-IR = Homeostasis Model Assessment of Insulin Resistance
EAH = Eating in the Absence of Hunger
Race/Ethnicity = 1 = non-Hispanic White, 0 = People of Color
p < .05
p < .01
p < .001
Tests conducted: Multivariate analysis of variance or binary logistic regression, as appropriate
Figure 2.
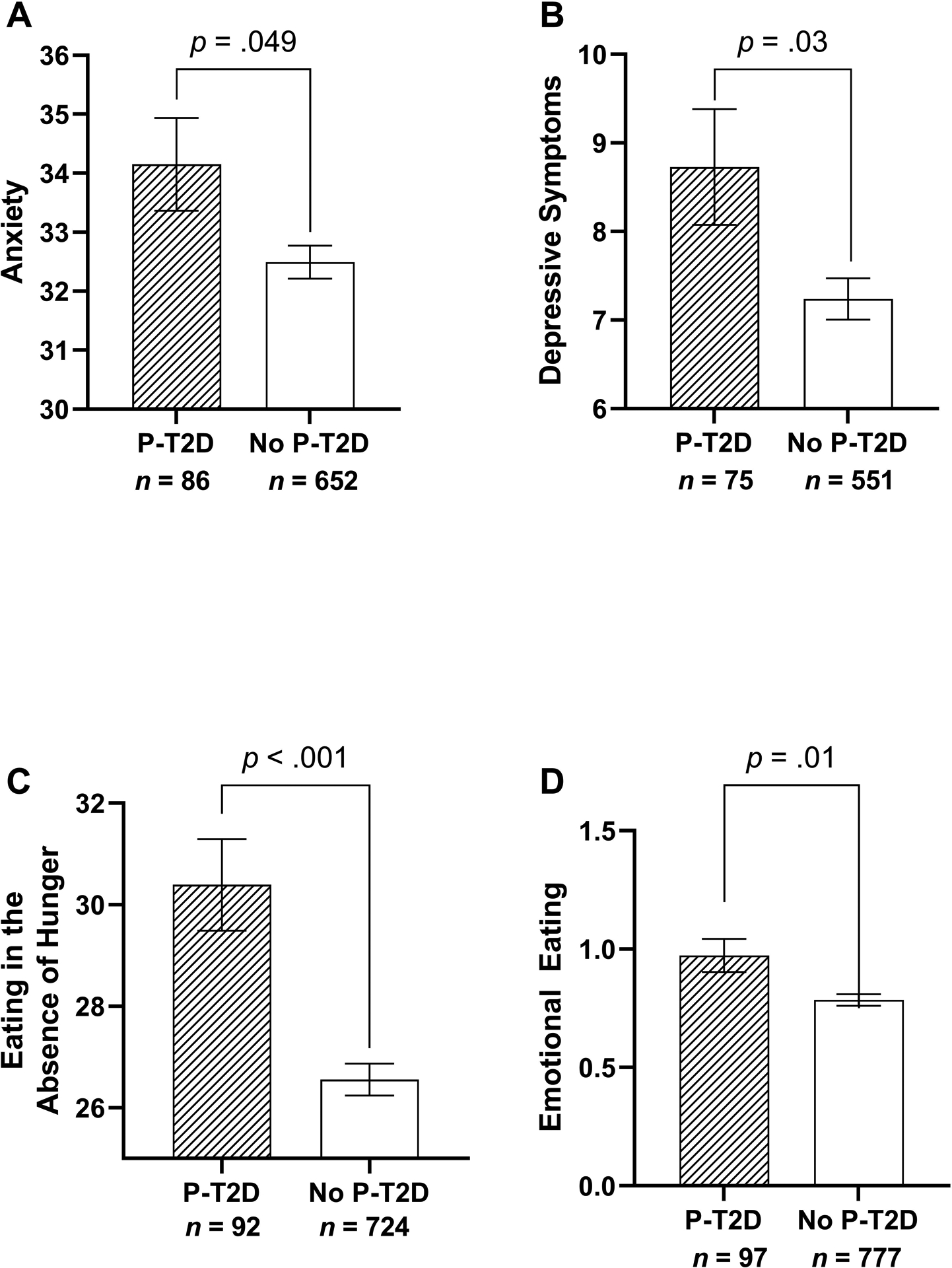
Associations of negative affect and disinhibited eating behaviors for children where 1 or both parents had type 2 diabetes (P-T2D) or for children where neither parent had type 2 diabetes (No P-T2D). Analyses were adjusted for age, sex, race/ethnicity, fat mass (kg), height, and insulin resistance using the homeostasis model assessment of insulin resistance (HOMA-IR) index. Compared to youth without a P-T2D, youth with a P-T2D self-reported greater a. anxiety (p = .049), b. depressive symptoms (p = .034), c. eating in the absence of hunger (p < .001), and d. emotional-eating (p = .012).
When analyses were repeated adjusting for intervention-seeking status, the relationships between P-T2D and anxiety (F(1, 729) = 2.22, p = .138, ηp2 = .003) and depressive symptoms (F(1, 617) = 0.71, p = .400, ηp2 = .001) became non-significant. Separating for intervention-seeking and non-intervention participants, neither model was significant for anxiety (intervention-seeking: p = .159, ηp2 = .01; non-intervention: p = .903, ηp2 < .001) or depressive symptoms (intervention-seeking: p = .682, ηp2 = .001; non-intervention: p = .490, ηp2 = .001).
Reported disinhibited eating behaviors
In models adjusting for age, sex, race/ethnicity, children with a P-T2D reported greater EAH (F(1, 811) = 17.48, p < .001, ηp2 = .02) and emotional-eating (F(1, 869) = 8.62, p = .003, ηp2 = .01) compared to children without a P-T2D. When adiposity and height were included as additional covariates, findings remained consistent for greater EAH (F(1, 809) = 15.52, p < .001, ηp2 = .02) and emotional-eating (F(1, 867) = 6.79, p = .009, ηp2 = .01) among children with a P-T2D. When HOMA-IR was included as a final additional covariate, results remained consistent for EAH (F(1, 808) = 15.85, p < .001, ηp2 = .02; Table 3; Figure 2c) and emotional-eating (F(1, 866) = 6.41, p = .012, ηp2 = .01; Table 3; Figure 2d).
Adjusting for intervention-seeking status, the relationship between P-T2D and EAH remained significant (F(1, 807) = 9.88, p = .002, ηp2 = .01), while the relationship between P-T2D and emotional-eating was attenuated (F(1, 865) = 3.09, p = .079, ηp2 = .004). For models separated for intervention-seeking and non-intervention participants, EAH findings remained significant for intervention-seeking (p = .016, ηp2 = .03), but was attenuated for non-intervention-seeking participants (p = .074, ηp2 = .01). For emotional-eating, the relationship became non-significant for intervention-seeking (p = .558, ηp2 = .001) and remained significant for non-intervention (p = .045, ηp2 = .01) participants.
Adjusting for age, sex, race/ethnicity, there were no significant group differences between children with and without a P-T2D in presence of LOC-eating (β = 0.14, p = .541, OR = 1.15). When adiposity and height were included, results remained non-significant (β = −0.14, p = .546, OR = 0.87). Likewise, when HOMA-IR was included as an additional covariate, there were no differences in results (β = −0.08, p = .739, OR = 0.92; Table 3). When analyses were repeated adjusting for intervention-seeking status, the relationship remained non-significant (β = −0.47, p = .077, OR = 0.63).1
Exploratory Analyses of Energy Intake
For the EAH test meal, adjusting for age, race/ethnicity, there were no significant differences in total energy intake (F(1, 95) = 0.10, p = .754, ηp2 < .01) or percentage of energy consumed from protein (F(1, 95) = 0.94, p = .335, ηp2 = .01), fat (F(1, 95) = .61, p = .436, ηp2 = .01), or carbohydrates (F(1, 95) = 1.53, p = .219, ηp2 = .02) between youth with and without a P-T2D. In models of total energy intake adjusting for age, race/ethnicity, height, fat mass (%), and lean mass (kg), there were no differences between groups (F(1, 91) = 0.06, p = .802, ηp2 = .001). When HOMA-IR was included as an additional covariate for models of total caloric intake and macronutrient content, results remained unchanged (ps >.283).
For the LOC-eating test meal, adjusting for age, sex, race/ethnicity, there were no significant differences in total energy intake (F(1, 194) = 1.99, p = .160, ηp2 = .01), percentage of energy consumed from protein (F(1, 194) = 0.47, p = .494, ηp2 = .002), fat (F(1, 194) = 0.17, p = .683, ηp2 = .001), or carbohydrates (F(1, 194) = 0.02, p = .885, ηp2 < .001) between youth with and without a P-T2D. In the model for total energy intake adjusting for age, sex, race/ethnicity, height, fat mass (%), and fat free mass (kg), there were no significant differences (F(1,191) = 0.65, p = .422, ηp2 = .003). When HOMA-IR was included as an additional covariate for models of total caloric intake and macronutrient content, results remained unchanged (ps > .423).
Discussion
This study aimed to explore whether youth with a P-T2D were more likely to report more psychological symptoms and disinhibited eating compared to those without a P-T2D as an initial step to elucidate psychological and behavioral factors that might confer risk for T2D. Findings suggest that youth with a P-T2D had higher anxiety, depressive symptoms, EAH, and emotional-eating above and beyond the contribution of known risk factors for T2D, including adiposity and IR. However, several findings were diminished when intervention-seeking status was considered, particularly for negative affect symptoms. Youth with a P-T2D may be at heightened risk of negative affect and disinhibited eating risk factors that directly contribute to and increase their biological risk for development of T2D compared to youth without a P-T2D. In contrast to hypotheses, reported LOC-eating was not different between children with a P-T2D and those without. Exploratory analyses revealed no relationship between having a P-T2D and objective EAH or LOC-eating, as measured by test meals.
While findings support previously reported cross-sectional links between depressive symptoms and increased IR in youth,25 and prospective data that depressive symptoms predict worsening IR,24 they should be interpreted cautiously since consideration of intervention-seeking status diminished findings. Should results be replicated, several possibilities may account for these relationships. In youth with higher weight, depressive symptoms are associated with poorer cardiorespiratory fitness,48 which is linked to IR.49 Depression may also promote IR through altered pathophysiological processes, such as chronic hyperactivity of the hypothalamic-pituitary-adrenal axis or inflammation within the central nervous system.50 While the current sample included youth of a broad age range and data were captured only at one time point, the mean age of the sample was 13 years, which is often when puberty occurs.51 The onset of puberty causes normative endocrine changes, related to both elevated depressive symptoms, especially among females,52 and increased IR.53
Although at least two prior studies found no relationship between anxiety and IR among youth,21, 25 youth with a P-T2D reported higher anxiety symptoms than those without a P-T2D. Findings persisted above and beyond the important contributions of child adiposity and IR, factors closely linked with a child’s genetic risk for T2D, yet they were attenuated when considering intervention-seeking status and appeared to be driven by those seeking intervention. It is possible that these associations may be due to the impact parental chronic illness has on children’s psychosocial well-being. Chronic illness in parents may result in psychological distress in offspring, including greater anxiety and depressive symptoms from an increase in daily hassles/responsibilities put on the child and perceived stress of parent’s illness.54 However, data regarding offspring’s well-being specifically surrounding parental T2D are limited and heterogeneous.55
Regarding disinhibited eating, both self-reported EAH and emotional-eating, but not LOC-eating, were higher among youth with a P-T2D. Adding to previous literature that binge-eating behaviors are linked to T2D,22, 23, 26 our findings support that disinhibited eating behaviors may be present prior to T2D diagnosis, suggesting they are part of the biological predisposition to the disease. Notably, both EAH and emotional-eating were assessed by questionnaire. Interview-based measures, such as the Eating Disorder Examination, tend to be more conservative than questionnaire methods, which are also more vulnerable to subjective bias.56 Indeed, youth who report higher scores on one questionnaire tend to also endorse higher scores on others, even when measuring different constructs.57, 58 Exploratory test meal analyses further bolster this possibility; when measured by objective intake in the laboratory, there were no differences between youth with and without a P-T2D for any type of intake.
Strengths of the current study include examination of a large and diverse sample of children with and without a parental history of T2D. All analyses were repeated adjusting for adiposity and, again, for IR, allowing for a more robust examination of the relationship between parental T2D and negative affect and disinhibited eating behaviors, above and beyond child physiological predictors. Additionally, we used DXA or Bod Pod to assess body composition and adiposity rather than BMIz. Limitations include use of self-report questionnaires for most of the dependent variables. Significant findings for self-report constructs (i.e., anxiety, depression, EAH, emotional-eating) should be interpreted with caution given that findings with neither interview nor objective test meal techniques, which are arguably more accurate methods of the constructs of interest, were significant. Moreover, findings were diminished or attenuated when considering intervention-status. As no clear pattern emerged across variables, findings may not be driven solely by intervention-seeking status, but rather that the combined sample provided enough power to detect significant relationships. To this end, our results require replication and we recommend caution when interpreting findings. The compiled sample was predominantly female (nearly 70%). Thus, it is not surprising that sex had an effect in several models, and study results should be interpreted within this context. Further, parents’ BMI, eating habits, and household food insecurity were not collected for all studies and thus were not considered in analyses. Energy intake analyses had small sample sizes of children with a P-T2D. Finally, our data were cross-sectional, limiting the ability to draw any causal conclusions between mood and disinhibited eating behavior risk factors and future IR and T2D development.
In conclusion, self-reported negative affect and disinhibited eating behaviors were higher among youth who were genetically predisposed to T2D. If replicated, providers who work with youth genetically predisposed to T2D might consider recommending interventions that address negative affect and disinhibited eating.
Research support:
This research was supported by the following sources: National Research Service Award 1F32HD056762 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (to LBS); NICHD Grant K99/R00HD069516 (to LBS); Uniformed Services University of the Health Sciences grant R072IC (to MTK); National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK080906-04 (to MTK); Intramural Research Program Grant 1ZIAHD000641 from the NICHD, with supplemental funding from the Division of Nutrition Research Coordination, the National Institute of Minority Health and Health Disparities, and the Office of Behavioral and Social Sciences Research (to JAY).
Footnotes
Publisher's Disclaimer: Disclaimer: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the National Institutes of Health, Uniformed Services University of the Health Sciences, or the United States Department of Defense.
All analyses were repeated excluding youth with two P-T2D and results were broadly consistent for all variables except for symptoms of anxiety, which had marginally insignificant changes when including adiposity and height as additional covariates (F(1, 718) = 3.15, p = .08, ηp2 = .004) and including HOMA-IR as a final additional covariate (F(1, 717) = 3.12, p = .08, ηp2 = .004).
Trial Registration: Clinical Trials.gov ID#: NCT00263536; NCT00680979; NCT01425905; NCT00001522; NCT00320177; NCT00631644; NCT02390765
Competing Interests: The authors declare no competing financial interests.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. The Journal of the American Medical Association. 2018;319(16):1723–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Divers J, Mayer-Davis EJ, Lawrence JM, Isom S, Dabelea D, Dolan L, et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths - Selected Counties and Indian Reservations, United States, 2002–2015. The Morbidity and Mortality Weekly Report. 2020;69(6):161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamman RF, Bell RA, Dabelea D, D’Agostino RB Jr., Dolan L, Imperatore G, et al. The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care. 2014;37(12):3336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornelis MC, Zaitlen N, Hu FB, Kraft P, Price AL. Genetic and environmental components of family history in type 2 diabetes. Hum Genet. 2015;134(2):259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. New England Journal of Medicine. 2017;376(15):1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham Offspring Study. Diabetes. 2000;49(12):2201–7. [DOI] [PubMed] [Google Scholar]
- 7.Lee CY, Ledoux TA, Johnston CA, Ayala GX, O’Connor DP. Association of parental body mass index (BMI) with child’s health behaviors and child’s BMI depend on child’s age. BMC Obesity. 2019;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstock RS, Trief PM, El Ghormli L, Goland R, McKay S, Milaszewski K, et al. Parental Characteristics Associated With Outcomes in Youth With Type 2 Diabetes: Results From the TODAY Clinical Trial. Diabetes Care. 2015;38(5):784–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Adamo E, Caprio S. Type 2 diabetes in youth: epidemiology and pathophysiology. Diabetes Care. 2011;34 Suppl 2(Suppl 2):S161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannon TS, Rao G, Arslanian SA. Childhood obesity and type 2 diabetes mellitus. Pediatrics. 2005;116(2):473–80. [DOI] [PubMed] [Google Scholar]
- 11.Brown AE, Walker M. Genetics of Insulin Resistance and the Metabolic Syndrome. Current Cardiology Reports. 2016;18(8):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demmer RT, Gelb S, Suglia SF, Keyes KM, Aiello AE, Colombo PC, et al. Sex differences in the association between depression, anxiety, and type 2 diabetes mellitus. Psychosomatic Medicine. 2015;77(4):467–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grigsby AB, Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. Prevalence of anxiety in adults with diabetes: a systematic review. Journal of Psychosomatic Research. 2002;53(6):1053–60. [DOI] [PubMed] [Google Scholar]
- 14.Khuwaja AK, Lalani S, Dhanani R, Azam IS, Rafique G, White F. Anxiety and depression among outpatients with type 2 diabetes: A multi-centre study of prevalence and associated factors. Diabetology and Metabolic Syndrome. 2010;2(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roupa Z, Koulouri A, Sotiropoulou P, Makrinika E, Marneras X, Lahana I, et al. Anxiety and depression in patients with type 2 diabetes mellitus, depending on sex and body mass index. Health Science Journal. 2009;3(1):32–40. [Google Scholar]
- 16.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49(5):837. [DOI] [PubMed] [Google Scholar]
- 17.Nouwen A, Winkley K, Twisk J, Lloyd CE, Peyrot M, Ismail K, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson JI, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, et al. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. The American Journal of Clinical Nutrition. 2010;91(6):1568–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenardy J, Mensch M, Bowen K, Green B, Walton J, Dalton M. Disordered eating behaviours in women with type 2 diabetes mellitus. Eating Behaviors. 2001;2(2):183–92. [DOI] [PubMed] [Google Scholar]
- 20.Raevuori A, Suokas J, Haukka J, Gissler M, Linna M, Grainger M, et al. Highly increased risk of type 2 diabetes in patients with binge eating disorder and bulimia nervosa. International Journal of Eating Disorders. 2015;48(6):555–62. [DOI] [PubMed] [Google Scholar]
- 21.Byrne ME, Tanofsky-Kraff M, Kelly NR, Grammer AC, Jaramillo M, Mi SJ, et al. Pediatric loss-of-control eating and anxiety in relation to components of metabolic syndrome. Journal of Pediatric Psychology. 2019;44(2):220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nip ASY, Reboussin BA, Dabelea D, Bellatorre A, Mayer-Davis EJ, Kahkoska AR, et al. Disordered Eating Behaviors in Youth and Young Adults With Type 1 or Type 2 Diabetes Receiving Insulin Therapy: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2019;42(5):859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinhas-Hamiel O, Standiford D, Hamiel D, Dolan LM, Cohen R, Zeitler PS. The type 2 family: a setting for development and treatment of adolescent type 2 diabetes mellitus. Archives of Pediatrics and Adolescent Medicine. 1999;153(10):1063–7. [DOI] [PubMed] [Google Scholar]
- 24.Shomaker LB, Tanofsky-Kraff M, Stern EA, Miller R, Zocca JM, Field SE, et al. Longitudinal study of depressive symptoms and progression of insulin resistance in youth at risk for adult obesity. Diabetes Care. 2011;34(11):2458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shomaker LB, Tanofsky-Kraff M, Young-Hyman D, Han J, Yanoff LB, Brady SM, et al. Psychological symptoms and insulin sensitivity in adolescents. Pediatric Diabetes. 2010;11(6):417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilfley D, Berkowitz R, Goebel-Fabbri A, Hirst K, Ievers-Landis C, Lipman TH, et al. Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care. 2011;34(4):858–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuczmarski RJ, RJ K, CL O, SS G, LM G-S, KM F, et al. 2000 CDC Growth Charts for the United States: methods and development: Vital and Health Statistics Series; 2002. [PubMed]
- 28.Ellis KJ. Human body composition: in vivo methods. Physiological Reviews. 2000;80(2):649–80. [DOI] [PubMed] [Google Scholar]
- 29.Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity. 2009;17(6):1281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva DR, Ribeiro AS, Pavão FH, Ronque ER, Avelar A, Silva AM, et al. Validity of the methods to assess body fat in children and adolescents using multi-compartment models as the reference method: a systematic review. Revista da Associação Médica Brasileira. 2013;59(5):475–86. [DOI] [PubMed] [Google Scholar]
- 31.Shank LM, Tanofsky-Kraff M, Kelly NR, Schvey NA, Marwitz SE, Mehari RD, et al. Pediatric Loss of Control Eating and High-Sensitivity C-Reactive Protein Concentrations. Childhood Obesity. 2017;13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robotham DR, Schoeller DA, Mercado AB, Mirch MC, Theim KR, Reynolds JC, et al. Estimates of body fat in children by Hologic QDR-2000 and QDR-4500A dual-energy X-ray absorptiometers compared with deuterium dilution. Journal of Pediatric Gastroenterology and Nutrition. 2006;42(3):331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 34.Spielberger CD. Manual for the state-trait anxiety inventory for children. Consulting Psychologists’ Press. 1973. [Google Scholar]
- 35.Kovacs M Children’s depression inventory: Manual: Multi-Health Systems North Tonawanda, NY; 1992. [Google Scholar]
- 36.Carey MP, Faulstich ME, Gresham FM, Ruggiero L, Enyart P. Children’s Depression Inventory: construct and discriminant validity across clinical and nonreferred (control) populations. Journal of Consulting and Clinical Psychology. 1987;55(5):755. [DOI] [PubMed] [Google Scholar]
- 37.Tanofsky-Kraff M, Ranzenhofer LM, Yanovski SZ, Schvey NA, Faith M, Gustafson J, et al. Psychometric properties of a new questionnaire to assess eating in the absence of hunger in children and adolescents. Appetite. 2008;51(1):148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanofsky-Kraff M, Theim KR, Yanovski SZ, Bassett AM, Burns NP, Ranzenhofer LM, et al. Validation of the emotional eating scale adapted for use in children and adolescents (EES-C). International Journal of Eating Disorders. 2007;40(3):232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vannucci A, Tanofsky-Kraff M, Shomaker LB, Ranzenhofer LM, Matheson BE, Cassidy OL, et al. Construct validity of the emotional eating scale adapted for children and adolescents. International Journal of Eating Disorders. 2012;36(7):938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fairburn CG, Cooper Z, O’Connor M. The eating disorder examination. International Journal of Eating Disorders. 1993;6:1–8. [Google Scholar]
- 41.Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the eating disorder examination with children: A pilot study. International Journal of Eating Disorders. 1996;19(4):391–7. [DOI] [PubMed] [Google Scholar]
- 42.Association AP. Diagnostic and Statistical Manual of Mental Disorders, 4th edition. Washington, DC: American Psychiatric Association; 1994. 1994. [Google Scholar]
- 43.Association AP. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013. 329–54 p. [Google Scholar]
- 44.Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Goldschmidt A, Yanovski SZ, et al. A multisite investigation of binge eating behaviors in children and adolescents. Journal of Consulting and Clinical Psychology. 2007;75(6):901–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shomaker LB, Tanofsky-Kraff M, Zocca JM, Courville A, Kozlosky M, Columbo KM, et al. Eating in the absence of hunger in adolescents: intake after a large-array meal compared with that after a standardized meal. American Journal of Clinical Nutrition. 2010;92(4):697–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, et al. Laboratory assessment of the food intake of children and adolescents with loss of control eating. American Journal of Clinical Nutrition. 2009;89(3):738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mirch MC, McDuffie JR, Yanovski SZ, Schollnberger M, Tanofsky-Kraff M, Theim KR, et al. Effects of binge eating on satiation, satiety, and energy intake of overweight children. American Journal of Clinical Nutrition. 2006;84(4):732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shomaker LB, Tanofsky-Kraff M, Zocca JM, Field SE, Drinkard B, Yanovski JA. Depressive symptoms and cardiorespiratory fitness in obese adolescents. Journal of Adolescent Health. 2012;50(1):87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmitz K, Jacobs D, Hong C, Steinberger J, Moran A, Sinaiko A. Association of physical activity with insulin sensitivity in children. International Journal of Obesity. 2002;26(10):1310–6. [DOI] [PubMed] [Google Scholar]
- 50.McIntyre RS, Rasgon NL, Kemp DE, Nguyen HT, Law CW, Taylor VH, et al. Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Current Diabetes Reports. 2009;9(1):51–9. [DOI] [PubMed] [Google Scholar]
- 51.Choices N Stages of puberty: what happens to boys and girls. 2018.
- 52.Angold A, Costello EJ, Worthman CM. Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28(1):51–61. [DOI] [PubMed] [Google Scholar]
- 53.Kelsey MM, Zeitler PS. Insulin Resistance of Puberty. Current Diabetes Reports. 2016;16(7):64. [DOI] [PubMed] [Google Scholar]
- 54.Pederson S, Revenson TA. Parental illness, family functioning, and adolescent well-being: a family ecology framework to guide research. Journal of Family Psychology. 2005;19(3):404–19. [DOI] [PubMed] [Google Scholar]
- 55.Landi G, Andreozzi MS, Pakenham KI, Grandi S, Tossani E. Psychosocial adjustment of young offspring in the context of parental type 1 and type 2 diabetes: a systematic review. Current Diabetes Reports. 2020;37(7):1103–13. [DOI] [PubMed] [Google Scholar]
- 56.Altman DR, Tanofsky-Kraff M, Shank LM, Swanson TN, Ramirez E, Moore NA, et al. Assessment of loss-of-control eating in healthy youth by interview and questionnaire. International Journal of Eating Disorders. 2020;53(5):780–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Field AE, Taylor CB, Celio A, Colditz GA. Comparison of self-report to interview assessment of bulimic behaviors among preadolescent and adolescent girls and boys. International Journal of Eating Disorders. 2004;35(1):86–92. [DOI] [PubMed] [Google Scholar]
- 58.Manassis K, Mendlowitz S, Menna R. Child and parent reports of childhood anxiety: differences in coping styles. Depression and Anxiety. 1997;6(2):62–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


