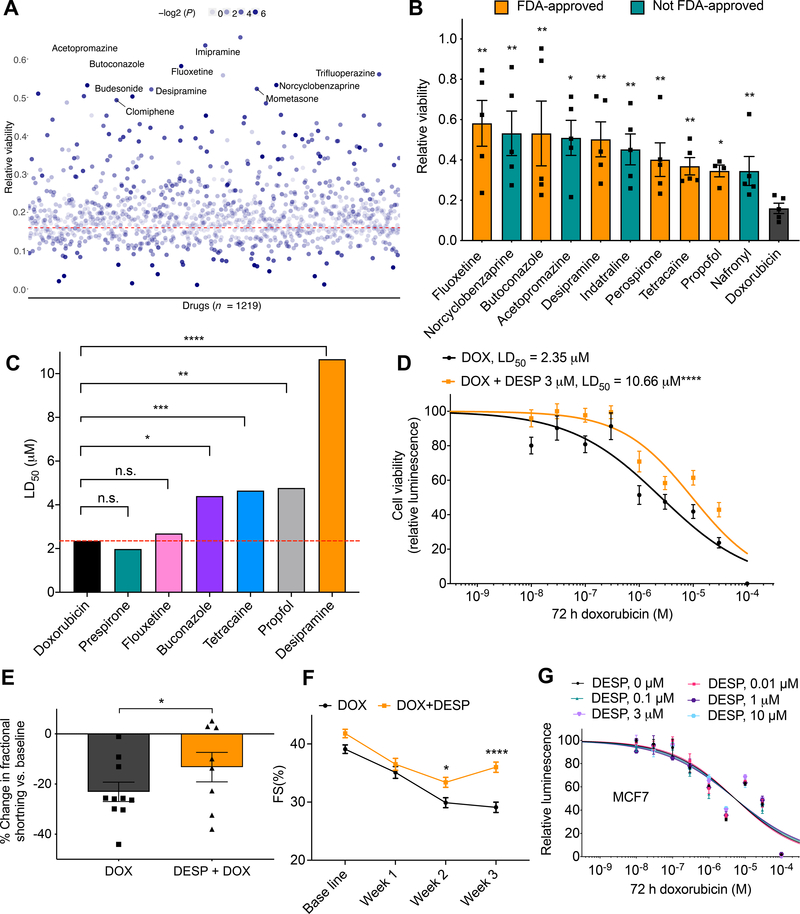
Figure 5. Multi-modality drug screening identifies desipramine as a novel effective cardioprotectant against DIC.
A, Prestwick drug library screening (n = 1219) in relation to DIC (n = 5). All drugs were used at 3 μM. Red dashed line represents cell viability 72 h post DOX (10 μM) treatment; the top ten significant cardioprotective drugs based on cell viability are labeled. B, Bar plot showing top ten significant cardioprotective (based on P value) compared to DOX alone (72 h, 10 μM) treated cells. Non–FDA–approved drugs are represented by teal bars. C, Further validation of top FDA-approved drugs (identified from the Prestwick library screening) against 10 log–doses of doxorubicin. LD50, median lethal dose. D, Effect of co-treatment of desipramine (3 μM) and doxorubicin (72 h) on hiPSC-CM viability [DOX (n = 42), DOX + DESP (n = 35)]. E, Percent change in ventricular fraction shortening (FS) normalized to baseline, after 3 weeks of doxorubicin treatment (3 mg/kg, ip, n = 10) compared co-treatment (n = 8) of desipramine (20 mg/kg/day, Alzet pump) and doxorubicin (3 mg/kg, ip) in mice. F, Ventricular fractional shortening at baseline, 1-, 2-, 3-weeks post treatment. G, Assessment of cell viability of MCF breast cancer cell line after 72 h of DOX and desipramine (DESP) cotreatment (n = 12–20). f = full independent experimental replicates, Error bars, s.e.m, *P < 0.05, **P ≤ 0.01, ***P < 0.001, ****P < 0.0001 by unpaired two-tailed Student’s t-test (A-C and E) and by ANOVA with post-hoc testing (F). For (D and G) log-logistic non-linear regression model was used to estimate the value of the four parameters, and t-statistic was used to test for significant difference in LD50 between different groups.