SUMMARY
How cells become specialized, or “mature”, is important for cell and developmental biology. While maturity is usually deemed a terminal fate, it may be more helpful to consider maturation not as a switch, but a dynamic continuum of adaptive phenotypic states set by genetic and environment programming. The hallmarks of maturity comprise changes in anatomy (form, gene circuitry, interconnectivity) and physiology (function, rhythms, proliferation) that confer adaptive behavior. We discuss efforts to harness their chemical (nutrients, oxygen, growth factors) and physical (mechanical, spatial, electrical) triggers in vitro and in vivo, and how maturation strategies may support disease research and regenerative medicine.
Keywords: Cell maturity, Tissue anatomy & physiology, Directed stem cell differentiation, Circadian rhythms, Energy metabolism, Biomaterials, Organoids, Microfluidic chips, Nanotechnology, Machine-tissue interfaces
eToc blurb Melton
Alvarez-Dominguez and Melton propose a framework for understanding cellular “maturity” that incorporates modern views on plasticity. Using this framework, they describe the known chemical and physical regulators of maturity and summarize approaches and progress in regulating maturity in experimental models and regenerative medicine
INTRODUCTION
Understanding how metazoan cells become specialized for specific tasks is a challenge for cell and developmental biology. Applying that knowledge can provide mastery over cell states for applications in regenerative medicine. While much progress has been made in making functional tissues from stem cells, a common problem is their immature phenotype. Often, the in vitro products only become fully functional following transplantation to a living host. If it were possible to crack what makes differentiated cells physiologically specialized, it may be possible to build—in the laboratory—replacement tissues to study and treat disease more effectively.
While many have referred to this issue, often called cell “maturity”, little agreement exists as to what makes cells mature. Some define maturity by anatomy; enucleated red blood cells are deemed mature after losing residual RNA and acquiring a disc shape. For others this is about physiology—how cells accomplish specialized functions; mature pancreatic beta cells, for instance, responding accurately to glucose levels for insulin secretion. And in other cases, gene markers come to the fore as a definition for mature cells, as with immune cell types. Consensus on what is meant by cell maturation may provide further clarity in the literature and help focus goals for clinical applications.
We review this subject through the lens of recent in vitro maturation efforts and cell plasticity during development, senescence, and disease. We propose that maturation is neither terminal nor unidirectional, but a continuum of adaptive states dynamically set by genes and the environment that achieve adaptive functioning (Figure 1). Within this framework, we examine hallmarks of maturity and their determinants across several cell types, survey efforts to harness them to foster maturity in vitro and combat loss in vivo, and discuss how maturation strategies and their products may support new applications.
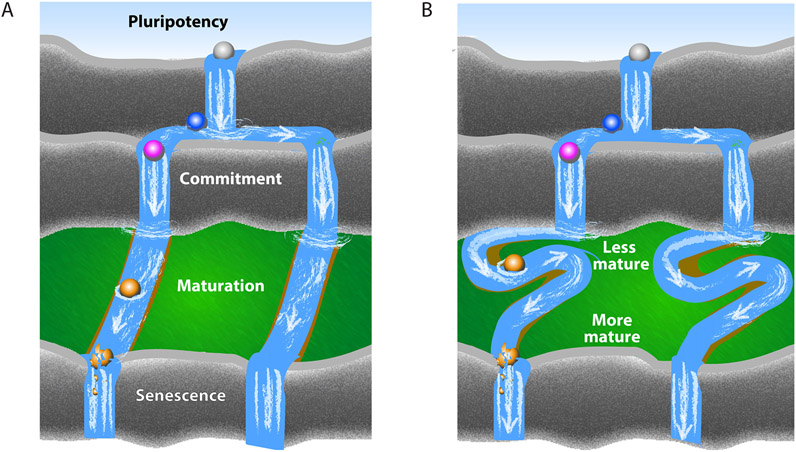
Figure 1. Cell Maturation.
(A) Maturation is classically understood as a unidirectional progression over time. Following commitment to a specific fate, differentiated cells mature by gaining specialized phenotypes, which decay during senescence. Cells are represented as spheres, their developmental stages by changing colors, and their differentiation time course by floating down the river.
(B) An alternative view of maturation as a fluid continuum of adaptive states: specialized cellular phenotypes are dynamically gained or lost in response to changes in the environment to attain maximally adaptive behavior.
Defining cell maturation
Following fate specification and determination, metazoan cells develop specialized physiological and morphological features to become fully functional. To the extent that these traits typify a fully grown organism, or a relatively stable part of its life, the specialized cell has been called “mature”. The term implies an “end state” of completed development, reached by an age-dependent unidirectional progression (Figure 1A). Yet, maturity may not be a development endpoint; cells may change in morphology and function with chronic or acute stress. Moreover, maturity may be attained independent of age. The progression of a cell from its formation to specialization can occur early in life (as with vascular, lung epithelia), repeatedly through life (as with red blood cells, intestinal epithelia), or in parallel with organismal development (as with cardiac, neuronal, pancreatic cells). And maturation need not be a one-way progression; specialized features are lost and regained as part of dedifferentiation in regeneration and disease.
Where does this leave the cell maturation concept? It may be useful to conceive of maturation as systematic processes that achieve maximally adaptive behavior. Viewed as an “adaptive state”, maturity reflects a time-dependent interplay of genetic and environment programming. Maturation is then closely related to development, but not synonymous. Development trajectories include entropic function declines that lack the systematic adaptive changes characterizing maturation. When we talk about maturation, we thus mean a development phase when specialized cell states are dynamically established in response to genetic and environment programming to attain maximal adaptation. Maturation defined in this way is neither terminal nor a one-way progression, but a fluid continuum of reversible adaptive states (Figure 1B). It pertains to fully differentiated cells and involves phenotypic plasticity, and it is independent of age: cell maturation can occur early, late, or throughout a lifespan.
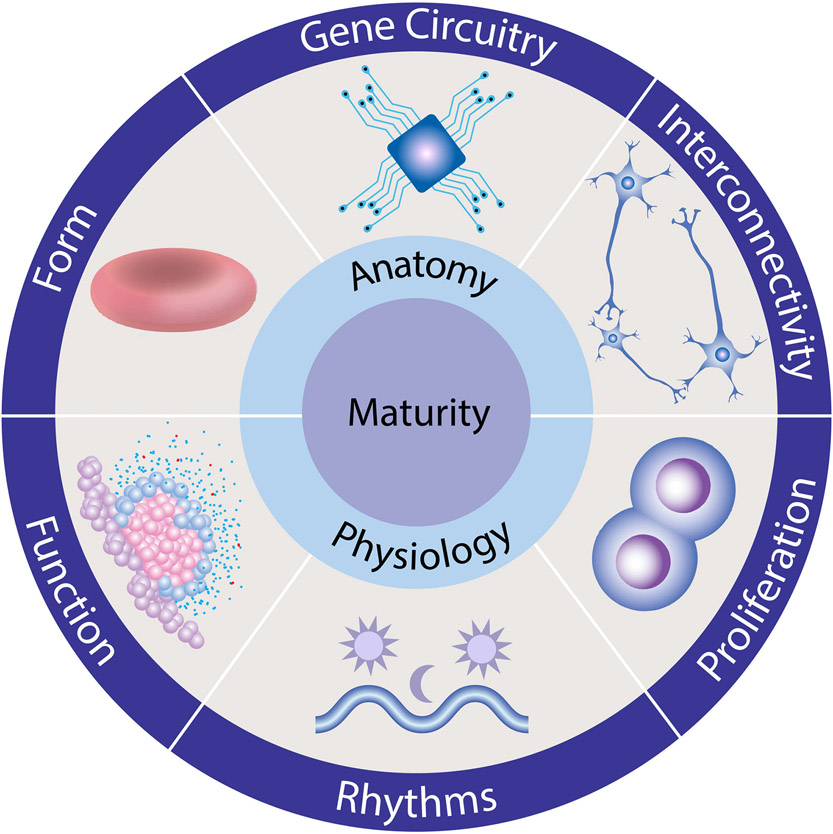
How should we assess maturity? Molecular or genetic criteria are yet to be fully enumerated in most cell types, whereas structural, functional characteristics are better defined. Maturity traits may be catalogued into anatomy (form, gene circuitry, interconnectivity) and physiology (function, rhythms, proliferation) hallmarks (Figure 2). Each represents an adaptation needed for specialization of differentiated cells. We consider these hallmarks and their determinants below, using select examples to illustrate their role in the maturation process, and highlight common features that may be used as criteria to assess maturity.
Figure 2. Maturity Hallmarks.
We suggest that most cell types acquire the same set of specialized traits during their maturation. These hallmarks are grouped into interrelated changes in anatomy (form, gene circuitry, interconnectivity) and physiology (function, rhythms, proliferation) that underlie phenotypic specialization.
Form
The generation of form is central to how cells become specialized for specific tasks. A mature red blood cell’s biconcave disc shape (Figure 3A) confers excess surface area relative to cell volume, enabling large deformations needed to transit through narrow capillaries. Red cell deformability is also determined by membrane elasticity, bestowed by transmembrane proteins tethered to a mechanically stable skeleton (Discher, 2000; Elgsaeter et al., 1986), and by cytoplasmic viscosity, set by ion and hemoglobin content (Mohandas and Gallagher, 2008; Renoux et al., 2019). Red cells turn spherical or crenate in hypotonic or hypertonic fluids, illustrating adaptability of their mature form to the environment. Underscoring its importance, altered form underlies hereditary red cell disorders (spherocytosis, elliptocytosis, sickle cell disease) (An and Mohandas, 2008; Delaunay, 2007). Form also decays with age, as senescent red cells lose the deformability needed to traverse capillaries, resulting in breakdown and removal from circulation (Mohandas and Gallagher, 2008).
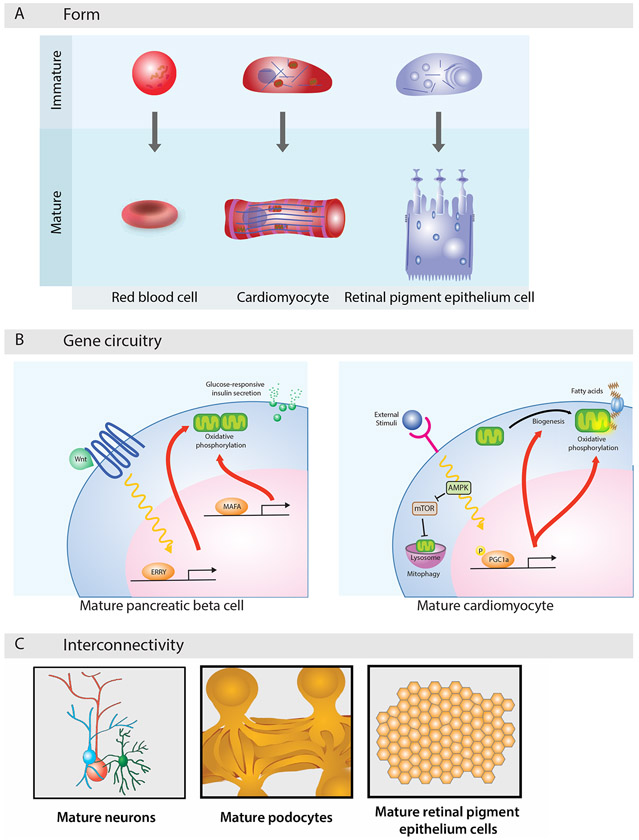
Figure 3. Anatomical Maturation.
(A) Cells morph to become specialized for specific tasks. Red blood cells turn into discs that withstand large deformations as they traverse the vasculature; cardiomyocytes morph into elongated rods that generate large contractile forces; and retinal pigment epithelial cells form polarized transport networks that support directional flow of cargo.
(B) Gene regulatory networks define maturation states. Transcriptional circuits set by MAFA and ERRγ in pancreatic beta cells enact mature coupling of insulin secretion to glucose oxidative metabolism, and post-transcriptional circuits mediated by AMPK and mTOR in maturing cardiomyocytes direct a metabolic shift toward fatty acid oxidation.
(C) Interconnectivity allows mature cells to execute complex tasks. Networks of synapsing neurons coordinate specialized sensory transduction; interdigitating kidney podocytes form slits that enable blood filtration; and tightly packed retinal pigment epithelial cells establish a semipermeable blood-retinal barrier.
Morphology also defines cardiomyocyte maturation. An elongated rod-like shape (Figure 3A) permits assembly of long aligned myofibrils that generate larger contractile forces (McCain and Parker, 2011; Siedner et al., 2003). Capacitance expands with surface area in maturing cardiomyocytes, raising the speed of action potential upstroke and propagation (Karbassi et al., 2020; Spach et al., 2004). And excitation-contraction coupling rises with increased sarcoplasmic reticulum volume and calcium content, and with formation of membrane invaginations that bring excitatory stimuli closer to contractile machinery (Bers, 2002; Ziman et al., 2010). Altered morphology underlies hereditary cardiomyocyte hypertrophy (Marian and Braunwald, 2017), while aging-associated hypertrophy, and excitation-contraction coupling decline, can lead to heart failure (Feridooni et al., 2015; Sheydina et al., 2011).
A third example is cell polarity, key for the retinal pigment epithelium. A morphology that facilitates polarized distribution of membrane channels, transporters, and receptors permits directional flow of water, nutrients, and waste products between choroid capillaries and photoreceptors (Figure 3A). Polarized traffic is supported in mature retinal pigment epithelial cells by basal membrane infoldings, which expand the surface area for transport, and by apical microvilli, which enwrap photoreceptors to facilitate retinoid exchange and signal-mediated phagocytosis (Lakkaraju et al., 2020; Strauss, 2005). As with heart and red cells, defects in specialized structural features of retinal pigment epithelial cells (ciliogenesis, polarized traffic) characterize congenital retinal disorders (May-Simera et al., 2018; Storm et al., 2020), while their decay with senescence characterizes age-associated macular degeneration (Sparrow et al., 2010).
Gene circuitry
Gene expression is a common criterion for calling a cell mature. In pancreatic beta cells, genetic circuits set by the MAFA and ERRγ transcription factors define maturation states (Figure 3B). MAFA programs glucose sensitivity of insulin release by binding genes for insulin and effectors of its glucose-sensitive secretion (Artner et al., 2010; Kataoka et al., 2002; Olbrot et al., 2002; Zhang et al., 2005). ERRγ programs higher insulin responsiveness to glucose by targeting genes tuning mitochondrial oxidative metabolism (Yoshihara et al., 2016). Inducing MAFA or ERRγ promotes these behaviors in immature beta cells (Aguayo-Mazzucato et al., 2011; Wang et al., 2007; Yoshihara et al., 2016), which informs efforts to make fully functional beta cells in vitro.
In cardiomyocytes, the HOPX transcription factor drives late maturation circuits. Inducing it prompts hypertrophic signaling, upregulating genes that promote growth and maturation in native (Kook et al., 2003) and in vitro-derived cardiomyocytes (Friedman et al., 2018). Interestingly, HOPX also marks maturity of native and in vitro-derived beta cells (Hrvatin et al., 2014; Veres et al., 2019), suggesting broad roles in steering maturational growth.
Post-transcriptional circuits can also steer maturation. AMPK and mTOR signaling mediate a shift from glycolysis to fatty acid oxidation that marks cardiomyocyte maturation (Figure 3B). Activated AMPK phosphorylates client proteins to promote fatty acid uptake, mitochondrial biogenesis and fission, and inhibits mTOR-dependent mitophagy suppression (Hang et al., 2018; Stuck et al., 2008). This enhances mitochondrial oxidative capacity with fatty acids as major substrates, enabling greater ATP production to support greater contractility (Karbassi et al., 2020). Interestingly, activating AMPK or inhibiting mTOR signaling fosters in vitro metabolic maturation of both cardiomyocytes (Garbern et al., 2020; Sarikhani et al., 2020) and pancreatic beta cells (Helman et al., 2020; Jaafar et al., 2019). Metabolic shifts illustrate adaptive states set by environmental conditions that not only accompany, but can drive maturation, as discussed below.
Interconnectivity
Emergent properties of complex systems are forged by the organization of their components. Higher-order network organization can thus determine cell maturation. Precise connectivity between neurons is key for specialized sensory transduction (Figure 3C). Following migration and outgrowth of axons and dendrites, neurons form premature synapses. Maturing neurons selectively expand or disassemble these structures, in response to spontaneous or stimulus-triggered activity, to form the precise local circuits that coordinate adult sensory processing (Katz and Shatz, 1996; Kirkby et al., 2013). Activity-dependent neural circuit refinement underlies learning and memory, illustrating lifelong maturational plasticity guided by the environment (Abbott and Nelson, 2000).
After growing polarized protrusions, kidney podocytes similarly interlink with each other to support blood filtration. Maturing podocytes wrap around glomerular capillaries by forming branching projections in an interdigitating pattern (Figure 3C). Interlinking projections shift from tight and adherens to porous intercellular junctions, which serve as filtration slits allowing selective retention of high-mass plasma components (Ichimura et al., 2017; Scott and Quaggin, 2015). This key glomerular filtration barrier feature is disrupted upon loss of podocyte interconnectivity due to injury or genetic mutation (Garg, 2018; Scott and Quaggin, 2015).
Connectivity also allows retinal pigment epithelial cells to establish an outer blood-retinal barrier. Maturing cells form tight junctions, shifting from fusiform to “cobblestone” organization (Figure 3C). Tight packing permits selective flow of solutes between blood and the neural retina (Rizzolo et al., 2011; Strauss, 2005). Directional flow of ions via active transport fosters the ionic environment that photoreceptors need to function properly. This increases electrical resistance across the cell monolayer, a key determinant of the mature retinal pigment epithelium barrier function that is lost in retinal diseases (Lakkaraju et al., 2020; Rizzolo et al., 2011).
Function
Specialized functioning defines mature behavior. Mature pancreatic beta cells respond selectively to supraphysiologic blood glucose with increased insulin secretion (Figure 4A). Selective responsiveness emerges gradually after birth, as beta cells adapt to a glucose- and oxygen-rich environment by increasing the glucose coupling and threshold for insulin secretion. These adaptations are determined by the beta cell’s affinity for glucose uptake, phosphorylation, and oxidation, and by its electrical excitability (Huang et al., 2018; Prentki et al., 2013; Rorsman and Ashcroft, 2018). Underscoring their importance, glucose metabolism and excitability defects underlie maturity-onset forms of diabetes (Fajans et al., 2001; Pipatpolkai et al., 2020; Rubio-Cabezas and Ellard, 2013). Disturbed nutrient or oxygen exposure during pregnancy or at birth alter the course of maturational adaptations, which are also remodeled by metabolic stress in diabetes, illustrating lifelong programming by the environment (Baeyens et al., 2016; Weir et al., 2001). Restoring mature glucose responsiveness in stressed beta cells thus presents an attractive opportunity to reverse pathology associated with obesity and diabetes.
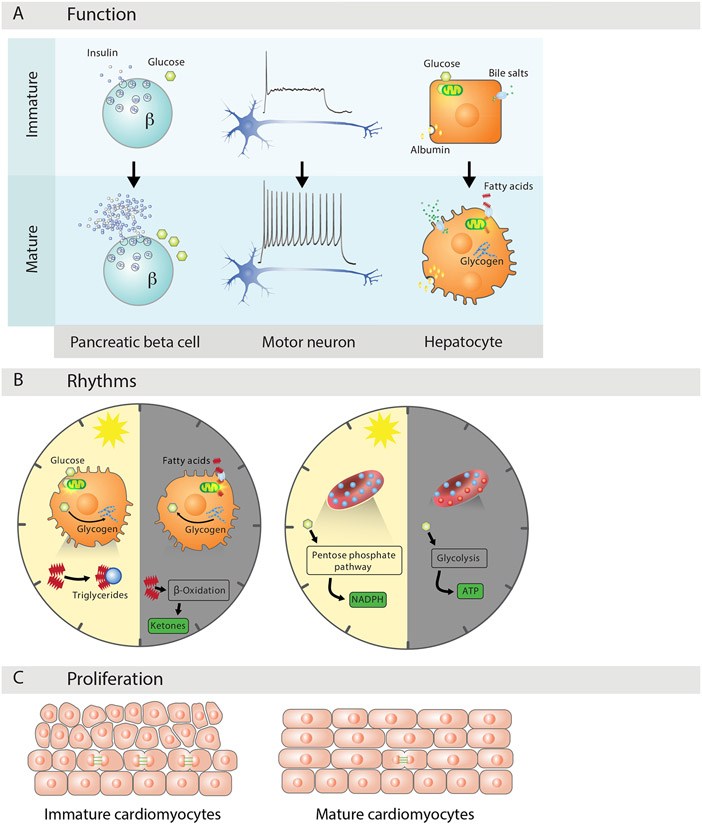
Figure 4. Physiological Maturation.
(A) Function specialization defines maturational change. Mature beta cells respond to glucose stimulation with increased selectivity and insulin release capacity; mature motor neurons respond to steady stimulation with repeated firing of decreasing frequency; and mature hepatocytes expand export of albumin and bile salts, metabolize glycogen, and oxidize lipids for energy.
(B) A rhythmic physiology is vital for maximally adaptive functioning. Hepatocytes consume glucose and synthesize glycogen and triglycerides with daytime feeding, and shift to fatty acid consumption, glycogen breakdown, and ketone synthesis during nighttime fasting. Mature red cells turn to anaerobic glycolysis as oxygen dips at night, and re-route flux to NAPH synthesis to reduce hemoglobin oxidation as it rises during the day.
(C) Proliferative adaptations underlie maturation states. Mature cardiomyocytes are mostly quiescent, as shifting to lipid metabolism for energy triggers cell cycle arrest due to increased oxidative DNA damage.
Metabolic specialization similarly defines maturity of hepatocytes. Their ability to process metabolites, bile, urea, serum proteins, toxic compounds is set by metabolic adaptations to postnatal feeding, oxygenation, and hormonal changes (Figure 4A). Postnatal intermittent feeding introduces fasting periods of high glucagon signaling that induce enzymes enabling fat oxidation, glucose synthesis, and glycogen breakdown (Bideyan et al., 2021). Shifting to an oxygen-rich environment furthers albumin, urea synthesis and the activity of transporters and cytochromes mediating detoxification (Kidambi et al., 2009; Tilles et al., 2001). And raised glucocorticoid and cAMP signaling foster specialization of gluconeogenesis and glycogen storage (Shin and Monga, 2013; Trefts et al., 2017). These adaptations allow hepatocyte-specific control of metabolic homeostasis, and their response to overnutrition is tightly linked to diabetes, fatty liver disease, and cardiovascular dysfunction (Bechmann et al., 2012; Trefts et al., 2017).
A third example is the electrical specialization of motor neurons. Mature motor neurons adapt to steady stimulation by gradually decreasing the frequency of electrical firing (Figure 4A), enabling sustained muscle contraction (Granit et al., 1963; Meehan et al., 2010). Upon stimulus termination, rebound membrane depolarization drives a bursting firing pattern, key for rhythmic muscle movement (Bertrand and Cazalets, 1998; Hinckley et al., 2005). Adaptive activity modulation is determined by intrinsic excitability and synaptic function (Grillner, 2003; Kanning et al., 2010). Excitability is tuned by transmembrane ion channels controlling membrane polarization, and thus its conductance and potential. Synapse function involves synthesis, exocytosis, and sensing of neurotransmitters for effective electrical signal propagation, enabling network-level activity. Both intrinsic excitability and synaptic efficacy undergo activity-dependent modulation, illustrating flexible adaptive states that are impaired in motor neuron diseases (Bertrand and Cazalets, 2013; Kanning et al., 2010; Rekling et al., 2000).
Rhythms
Synchronizing cellular processes to energetic rhythms is vital for maximally adaptive behavior. Mature hepatocytes tune their physiology to circadian feeding-fasting cycles (Figure 4B) (Bideyan et al., 2021). During daytime feeding, they consume glucose for energy, storing the excess as glycogen, while turning dietary and newly synthesized fatty acids into triglycerides for export. With nighttime fasting, they shift to producing glucose, via glycogen breakdown and neogenesis, and instead consume fatty acids for energy and to make ketones for export. Glucose/lipid consumption-production rhythms are enforced by transcriptional feedback control between circadian integrators and metabolic effectors (Bass and Takahashi, 2010), and are entrained by food intake periods (Damiola et al., 2000; Sinturel et al., 2021; Stokkan et al., 2001). Alignment of the mature hepatocyte’s clock with meal timing optimizes clearing and processing of circulating nutrients, while chronic misalignment can lead to obesity, metabolic syndrome, and diabetes (Bass and Lazar, 2016; Panda, 2016).
Non-transcriptional rhythms also govern mature physiology. Despite lacking a nucleus or mitochondria, mature red blood cells sustain daily metabolic cycles to manage oxidative stress (Figure 4B). At night, when blood oxygen dips, glucose consumption to make ATP via anerobic glycolysis peaks (Mortola and Seifert, 2002; Sinturel et al., 2021). During the day, when oxygenation is highest, flux is re-routed to the pentose phosphate pathway to yield NADPH, needed to reduce reactive oxygen species. This causes 24-hour rhythms in glycolysis-generated ATP driving ion transport and membrane depolarization, and in the activity of peroxiredoxin enzymes mitigating hemoglobin oxidation (Cho et al., 2014; Henslee et al., 2017; O'Neill and Reddy, 2011). Metabolic and reduction-oxidation cycles persist without new RNA or protein synthesis in red blood cells, and disruptions due to genetic mutation or senescence result in anemia (Kuhn et al., 2017; Mohanty et al., 2014).
Proliferation
Cell proliferation and maturation are generally anticorrelated during development. After birth, most cardiomyocytes exit the cell cycle as part of metabolic maturation (Figure 4C). Shifting to fatty acid oxidation elevates reactive oxygen species that damage DNA, triggering cell cycle arrest via suppression of cyclin and cyclin-dependent kinases (Mohamed et al., 2018; Puente et al., 2014). A final DNA synthesis round without cell division causes increased ploidy and size, hallmarks of mature cardiomyocytes (Laflamme and Murry, 2011) (Figure 4C). Notably, this adaptive state is reversible, as quiescent cardiomyocytes can reenter the cell cycle with injury and aging (Bergmann et al., 2009; Karbassi et al., 2020).
Quiescence plasticity also distinguishes mature beta cells. Their self-replication rate in adults is slow (<1%/day), but increases upon metabolic stress. This adaptive ability develops after postnatal weaning to a carbohydrate-rich diet (Jacovetti et al., 2015; Stolovich-Rain et al., 2015). Maturing beta cells adjust to this nutritional change by increasing dependence on glucose as fuel for oxidative phosphorylation, and by restraining basal in favor of glucose-coupled replication, as with insulin secretion. Beta cell replication in response to metabolic stress depends on mitogenic glucose responsiveness, and loss of this adaptive trait of mature beta cells contributes to obesity-linked diabetes (Butler et al., 2003; Dor et al., 2004; Porat et al., 2011).
Like other neurons, mature motor neurons are postmitotic. Exit from the cell cycle is intrinsic to the neuronal differentiation program set by developmental patterning signals (Davis-Dusenbery et al., 2014). These activate bifunctional regulators that simultaneously foster differentiation, by inducing proneural genes, and cell cycle arrest, by suppressing cyclins and activating proarrest proteins (Bertrand et al., 2002; Politis et al., 2008). Lifelong cell cycle arrest is critical for the mature motor neuron phenotype, and cell cycle reentry, following injury or neurodegeneration, can cause fatal vulnerability (Herrup and Yang, 2007; Marlier et al., 2020).
In summary, cell maturation involves complex adaptations that may be reduced to a small set of underlying themes (Figure 2). These comprise interrelated changes in anatomy and physiology that respond to genetic and environment programming to confer maximally adaptive behavior. The hallmarks of anatomical (Figure 3) and physiological maturation (Figure 4) are shared across diverse cell types, and may thus be used as general criteria to assess maturity, while their disruption or decay can underlie numerous pathological conditions.
Harnessing cell maturation for research and clinical applications
How can we apply knowledge of cell maturation? Realizing cell maturation in the laboratory will permit modeling human development beyond its earliest stages, and may yield replacement cells and tissues for disease treatment and drug screening. How gene-environment interactions steer adaptive change in health and disease will be best studied using maturation-capable models. Drug discovery, safety and efficacy studies will likely benefit from screening products that closely recap the physiology of endogenous cell and tissue targets. And patients receiving mature cell transplants may experience faster and more durable therapeutic benefits.
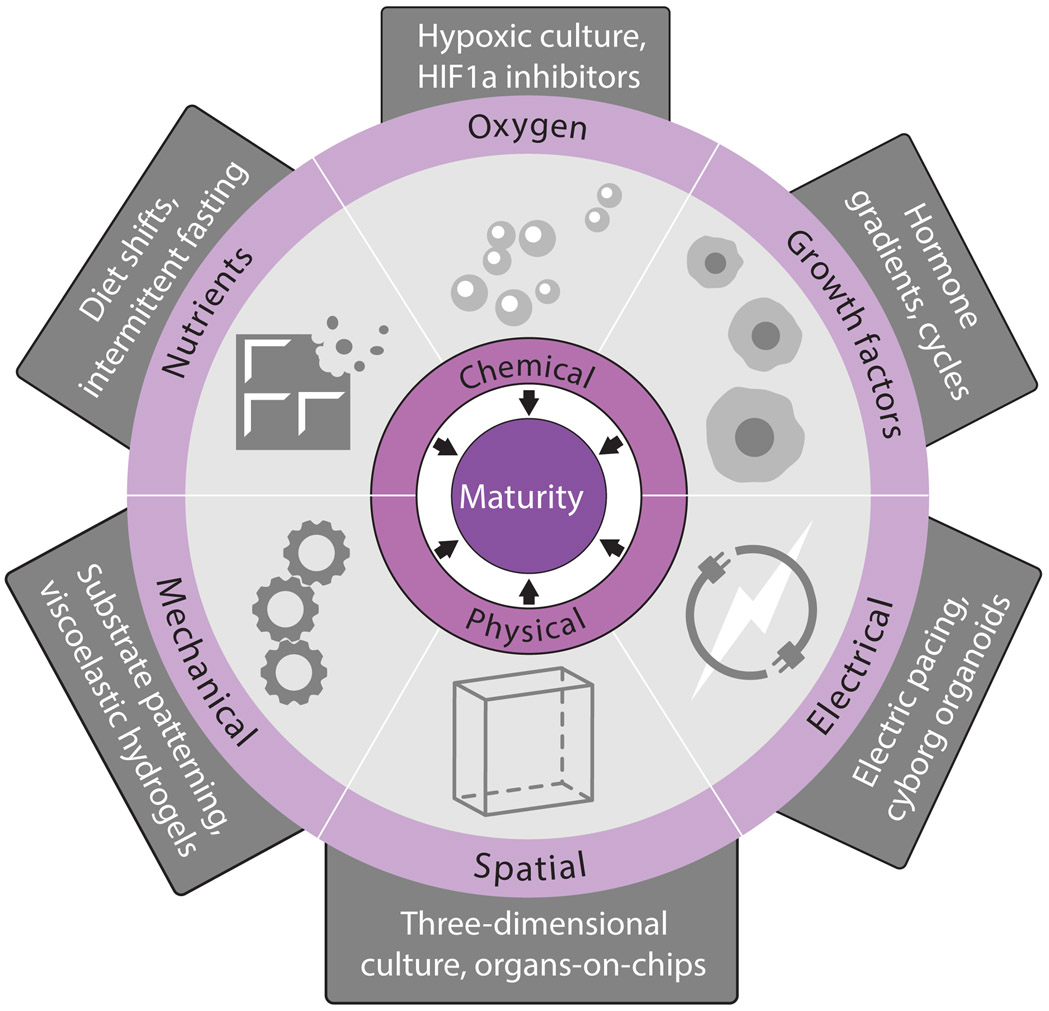
Harnessing cell maturation demands understanding its causes. Beyond delineating maturity states, recent studies have advanced knowledge of the cues instructing them. These may be organized into chemical (nutrients, oxygen, growth factors) and physical (mechanical, spatial, electrical) triggers (Figure 5). Below, we consider how such triggers can be manipulated to elicit phenotypic maturity or to prevent its loss, using select examples to illustrate how these applications may be useful (Figure 6).
Figure 5. Maturity Triggers.
We propose that cell maturation is instructed by cues from the environment that may be grouped into chemical (nutrients, oxygen, growth factors) and physical (mechanical, spatial, electrical) triggers. Manipulations to the culture environment in vitro and in vivo enable control over each of these triggers. The manipulations listed are but illustrative examples of emerging efforts to gain mastery over maturational development on multiple fronts.
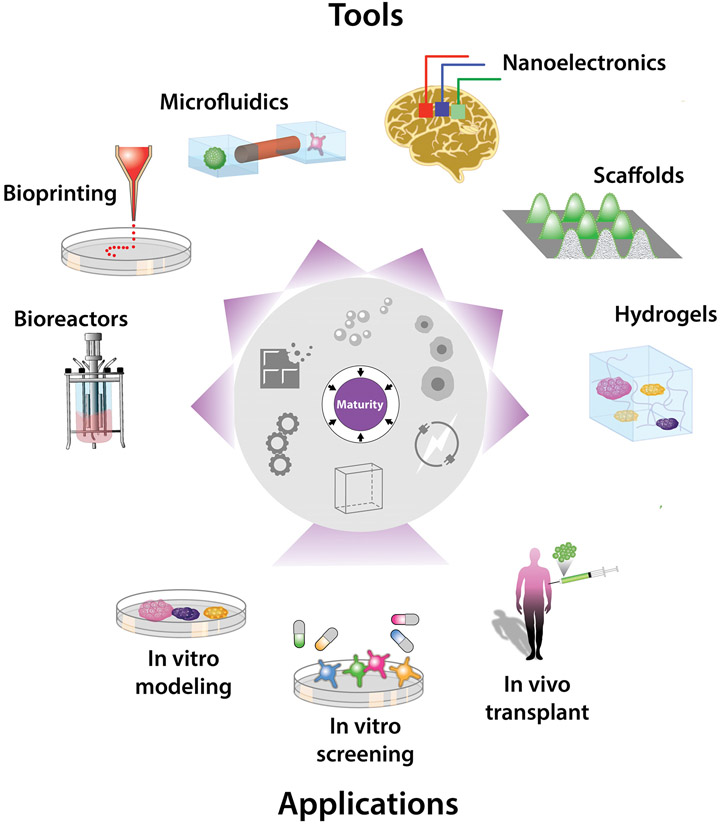
Figure 6. Harnessing Maturation for Research and Clinical Applications.
A growing toolbox makes it increasingly possible to harness cell maturation for applications in basic research, pharmacology, and regenerative medicine. The toolbox includes biomaterials and electromechanical devices offering standardized, scalable, and dynamic control over the physical and chemical environment, key for consistent and accurate mature cell and tissue production. Applications include modeling human development, physiology, and disease; discovering and testing therapeutic drugs and biologicals; and replacing lost, damaged, or aged tissues.
Harnessing chemical maturity triggers
Nutrients, oxygen, and growth factors in the environment trigger physiologic maturation (Figure 5). This logic has been applied to cells derived in vitro from pluripotent stem cells, which often function like fetal or neonatal but not adult counterparts. Mimicking the postnatal shift to a lipid-rich diet fosters adult-like features in human stem cell-derived cardiomyocytes. Mature form, gene circuitry, function, and proliferation hallmarks (Figures 3A and 4C) are promoted by fatty acid supplementation, and hindered by glucose (Correia et al., 2017; Mills et al., 2017; Nakano et al., 2017). Dietary manipulations (amino acid, serum deprivation) also promote adult-like traits in pancreas beta cells derived from human stem cells (Helman et al., 2020; Velazco-Cruz et al., 2019; Veres et al., 2019). A third example is supplementation with vitamin K2 or lithocholic acid (an intestinal flora metabolite) promoting fetal and stem-cell derived hepatocyte maturity (Avior et al., 2015). These cases illustrate how environment nutrients/metabolites steer maturation. How nutritional shifts are transduced into the nucleus to program genetic change, and the role of energy metabolism in this process, remain unclear.
Oxygenation also steers maturational change. The shift to autonomous breathing at birth increases oxygen levels, suppressing hypoxia signaling. Emulating adult oxygen levels improves the generation of hepatocyte-like cells from human stem cells (Si-Tayeb et al., 2010) and prevents adult hepatocytes from losing their mature phenotype in culture (Guo et al., 2017). While suppressing hypoxia signaling, via HIF1a inhibition, recreates the postnatal switch from glycolytic to oxidative metabolism in stem cell-derived cardiomyocytes, promoting adult-like anatomy and physiology (Gentillon et al., 2019; Hu et al., 2018). Inhibiting HIF1a thus presents an attractive opportunity to rescue delayed tissue maturation caused by hypoxic injury at birth.
Growth factors are another readily pliable maturational cue. Glucocorticoids, which rise around birth, promote specialized physiology and morphology in both fetal (Kamiya et al., 2001; Rog-Zielinska et al., 2015) and stem cell-derived (Parikh et al., 2017; Zhu et al., 2014) hepatocytes and cardiomyocytes. Thyroid hormone, which rises postnatally, has similar effects in both fetal and stem cell-derived cardiomyocytes (Chattergoon et al., 2012; Lee et al., 2010) and beta cells (Aguayo-Mazzucato et al., 2015; Aguayo-Mazzucato et al., 2013). How these effects occur is emerging, and likely involves central metabolic modulators (Rog-Zielinska et al., 2015; Takano et al., 2013). Administering growth factors (or synthetic analogs), and manipulating their transducers and effectors, is thus a major focus of in vitro cell maturation efforts. Greater understanding of how these pathways tune maturity will likely benefit from detailed profiling of the gene circuits active at distinct cell maturity states by single-cell technologies.
Not just the nature, but the timing of chemical cues governs mature behavior. Nutrients, oxygen, and hormones are not constant, but fluctuate with sleep-wake and feeding-fasting cycles (Bass and Takahashi, 2010). Harnessing conditions promoting maturity thus demands recreating physiologic rhythmicity. Introducing daily glucose, amino acid, or insulin fluctuations fosters mature gene circuitry, rhythms, and function hallmarks (Figure 2) in stem-cell derived islets, accelerating their function and ability to reverse diabetes in mice upon transplant (Alvarez-Dominguez et al., 2020; Wang et al., 2020). The feeding/fasting entrainment imparts metabolic cycling, via chromatin remodeling and rhythmic synthesis of circadian clock and energy metabolism factors, enabling mature insulin responses. A circadian dietary rhythm may thus help counter neonatal diabetes caused by immature beta cell function (Hattersley et al., 2018; Rubio-Cabezas and Ellard, 2013). How circadian, nutrient, and gene programming interact during maturation is unclear, but a likely link is via clock-regulated synthesis of metabolites involved in both chromatin and clock protein modification (Bedont et al., 2020; Rutter et al., 2002). Circadian entrainment also fosters mature behavior in adult islets that lost their mature phenotype in culture (Alvarez-Dominguez et al., 2020), illustrating dynamic reprogramming of maturity by the environment. Circadian behavioral or chemical interventions may thus help rescue mature islet function lost in diabetic adults.
With better spatiotemporal control over chemical maturity triggers, it may be possible to direct precise maturation trajectories in engineered/explanted tissue (Figure 6). Microfluidics and synthetic hydrogels enable patterning or timed release of nutrients, oxygen, growth factors (Brennan et al., 2014; Darnell and Mooney, 2017). These may be harnessed to better model endogenous development and physiology, and to improve integration, vascularization, immunomodulation, and innervation of implanted cell-based products.
Harnessing physical maturity triggers
Mechanical, spatial, and electrical cues are key triggers of maturational adaptations (Figure 5). Solid tissues sense forces that steer specialization. When mesenchymal stem cells differentiate on substrates mimicking the stiffness of brain, muscle, or bone, their products gain phenotypes corresponding to the tissue whose mechanical properties were experienced (Engler et al., 2006; Roberts et al., 2016). Human stem cell-derived cardiomyocytes develop specialized contractility, genetic circuitry, and structural features when cultured on gels approximating adult cardiac tissue stiffness (Feaster et al., 2015; Martewicz et al., 2017). Culture on micropatterned substrates similarly enforces cardiomyocyte elongation, boosting contractility, mitochondrial content, and conductance for both fetal (Kim et al., 2010) and stem cell-derived cardiomyocytes (Rao et al., 2013; Ribeiro et al., 2015). Fluid mechanics also shape maturity. Shear and osmotic stress rise as maturing red blood cells enter the circulation, driving changes in ion and water permeability that set internal viscosity and a discoid shape (Larsen et al., 1981; Renoux et al., 2019). Blood flow is also critical for the mature arterial cell phenotype (Chong et al., 2011), and is harnessed in microfluidic chips to prompt maturation of stem cell-derived endothelial cells (Homan et al., 2019; Smith et al., 2018). How cells sense and adapt to external forces is best understood in adherent tissue, and involves mechanosensitive machinery (integrins, syndecans, ion channels, cytoskeletal proteins) and transcription effectors like YAP (Camargo et al., 2007) that establish mechanical memory via epigenome changes (Chaudhuri et al., 2020; Humphrey et al., 2014).
Not only static (stiffness, topography, stress) but dynamic tissue mechanics (plasticity, deformation, stress relaxation) steer cell phenotypes. Leveraging control over these properties, via advances in dynamic materials, can help harness cell maturity states. Viscoelastic hydrogels with fast stress relaxation foster mature form, gene circuitry, and proliferation in encapsulated chondrocytes, promoting formation of an interconnected cartilage-like matrix (Lee et al., 2017). This informs the design of FDA-approved hydrogel-based applications such as autologous chondrocyte implantation, engineered skin grafts, and bone regeneration devices (Chaudhuri et al., 2020). Porous hydrogels analogously sustain glucose-responsive insulin function of encapsulated stem cell-derived beta cells, enabling long-term diabetes reversal upon transplant into mice, while protecting from immune attack (Vegas et al., 2016; Yang et al., 2021). Future uses for dynamic biomaterial mechanics include the gradual or rhythmic activation of forces instructing defined maturation trajectories in engineered tissue constructs.
Spatial cues can also mold maturation states. Cell-cell contacts and diffusible signals allow cells to sense and adapt to the environment geometry and composition. Keeping a mature phenotype in adult chondrocytes, or eliciting one in stem cell-derived cardiomyocytes, is guided by culture dimensionality (von der Mark et al., 1977; Werley et al., 2017). Three-dimensional culture allowing cells to self-organize is most successful in offering a maturation-permissive spatial milieu. Self-guided rather than imparted assembly yields stem cell-derived organoids that better recap the anatomy and physiology of eye, brain, heart, and kidney tissue (Karbassi et al., 2020; Little and Combes, 2019; Sasai, 2013; Velasco et al., 2020). These products fail to recap maturation beyond birth, however, but mature to adult phenotypes when transplanted in vivo. Cues derived from proximity or contact with support tissues may thus be needed for maximally adaptive behavior. Co-culture with endothelial cells or fibroblasts indeed fosters cardiac, liver, and islet cell maturity (Berger et al., 2015; Dunn et al., 2019; Kojima, 2014; Lee et al., 2015). How tissue-tissue crosstalk fosters maturity is beginning to emerge, and informs the design of multi-tissue three-dimensional culture systems. These include organs-on-chips and multilineage organoids, which can mimic complex spatial and dynamic traits of organ systems. Organs-on-chips permit spatiotemporal control of diffusible signals, via microfluidics, and of tissue-tissue contacts, via chip architecture (Esch et al., 2011; Huh et al., 2011). However, they offer limited scalability for high-throughput applications, like drug or genetic screening, and can only model known maturational processes. Multilineage organoids, by contrast, can be made at scale, and follow intrinsic self-assembling programs of greater physiologic fidelity, but suffer from limited environmental control and stochastic variability (Lancaster and Knoblich, 2014; Sasai, 2013).
Realistic mature physiology may be attainable by blending microfluidics and organoids. Organoids-on-chips recreate complex organization within and between tissues, like arterial, renal, respiratory tubes that transport plasma, nutrients, air to connecting tissues or to the outside world, allowing blood flow, renal filtration, respiration (Hofer and Lutolf, 2021; Sharma et al., 2020). This may be exploited for systemic developmental, physiological, and pharmaceutical studies, or to study pathologic cross-tissue interactions underlying autoimmune, neuromuscular, gastrointestinal disorders. It may also be possible to combine micro-physiological systems with biomaterials to ultimately attain mature replacement tissues that are vascularized and innervated. Bioactive scaffolds can direct morphogenesis to mature stages, and facilitate engraftment and long-term survival and function of implanted replacements for lost, damaged, or aged tissue (Darnell and Mooney, 2017). Bioprinting, the three-dimensional deposition of cells, cues, and biomaterials by mechanical printers (Moroni et al., 2018), may further enhance consistent and accurate tissue fabrication, including patient-tailored tissue for personalized toxicology or replacement therapy.
Electrical cues are also emerging as maturity triggers. Ion fluxes, set by ion transporters in all cell types and communicated via cell-cell junctions, instruct cell form and behavior. Electrical field stimulation of cultured neonatal cardiomyocytes (Radisic et al., 2004) and engineered bone tissue (Leppik et al., 2018) induces mature form and interconnectivity. Similar effects are seen in human stem cell-derived cardiac tissue (Eng et al., 2016; Nunes et al., 2013), whose maturation is accelerated further by combining mechanical stress with electric pacing to mimic endogenous cardiac stretching and contraction (Ronaldson-Bouchard et al., 2018; Ruan et al., 2016). Dynamic stimulation (varying pacing frequency to follow an ‘intensity training’ regimen) triggers greater maturation than static pacing, promoting adult-like form, gene circuitry, and function hallmarks (Ronaldson-Bouchard et al., 2018). This illustrates how maturation is steered by a dynamic environment, as with feeding-fasting training. How electromechanical stimuli program cardiac maturity is unclear, but it does not seem to require accompanying cell volume or transcriptional changes (Ruan et al., 2016). Ion transporters and electrical synapses are gated posttranslationally, and may program cell behavior independent of gene expression. Greater understanding of how electrical signals shape mature cell states will thus benefit from bioelectrical in addition to biochemical profiling.
Recent technological advances enable not just sensing, but actuating electrical circuits in single cells with high precision. Optogenetics enables light-induced control of ion transporters (Jakesova et al., 2019), which may be used to precisely instruct maturational change. Advances in nanoscale biomaterials enable miniaturization of bioelectronic interfaces, allowing stimulation of individual cells and membrane proteins without gene engineering. These include conducting nanomaterials and soft nanoelectronics. Conducting nanomaterials (nanotubes, nanowires, nanoparticles) that contact or traverse the cell membrane can directly alter its electric potential with high spatiotemporal precision (Noy, 2011; Tian and Lieber, 2019). Soft nanoelectronics, like stretchable mesh nanoelectrode arrays, allow stimulating single cells with millisecond resolution, and may be seamlessly integrated into “cyborg” organoids to direct maturation through systematic stimulation (Li et al., 2019; Tian et al., 2012).
The advent of injectable, tissue-like nanoelectronics heralds new opportunities for modulating cell maturity in vivo, via minimally invasive cyborg implants (Duan et al., 2013; Liu et al., 2015). These may incorporate traditional biomaterials, like hydrogel scaffolds, to better mimic physical properties of native tissue (Dvir et al., 2011; Feiner and Dvir, 2017). Combining sensing and actuation capabilities in wearable implants, though technically challenging, may ultimately enable closed-loop control of phenotypic maturity. This would be much like closed-loop control in current artificial pacemaker, pancreas, and neuromodulatory devices, but applicable beyond excitable tissues. In the future (Figure 6), one may exploit such tissue-electronics interfaces to study physiologic maturation, to treat the loss of phenotypic maturity in damaged or aged tissue, and to build fully functional replacements for any lost tissue.
In summary, manipulating cell and tissue maturation is actionable through a small set of physiologic triggers (Figure 5). These comprise chemical and physical cues that instruct maturational adaptation by their nature and timing. Harnessing them is viable via spatiotemporal modulation of the culture environment’s composition, design, and electromechanical properties. A growing toolbox of biomaterials and miniaturized interfaces enable precise, simultaneous control over these features in vitro and in vivo, potentiating a new generation of basic research, pharmacology, and regenerative medicine applications (Figure 6).
Synthesis
Cell behavior is fundamentally adaptive. Maturity is determined not by age, but by anatomical and physiological changes that respond to environmental and genetic inputs for maximal adaptation. Better understanding of the mechanisms enabling these changes is required (Box 1) to develop mastery over cell maturation, a key step toward greater utility of stem cell-derived tissue constructs. Harnessing the triggers and mechanisms of maturation may be achieved by tuning the chemical and physical environment, as well as their time-dependent variation. Precise spatiotemporal control over these inputs is increasingly attainable through advances in materials and electrochemical engineering. This general approach may make it possible to not just recreate, but improve upon nature’s maturation mechanisms, facilitating consistent and scalable engineering of cell products for screening and toxicology, not to mention to better model human organogenesis and disease. In the clinic, patients will benefit from receiving faithful replacements for lost or damaged tissues that attain maximal engraftment, survival, and function via tunable maturation. It might someday be possible to use such products not only to replace tissues, but to enhance the function of intact ones. Harnessing cell maturation will thus unlock worthwhile opportunities to prevent disease and to restore, or even improve human health.
Box 1. Unanswered Questions.
How do gene-environment interactions steer maturation trajectories in health and disease?
How are nutrient shifts communicated to the nucleus to program gene circuitry changes?
What role does energy metabolism play in establishing maturity states?
How do circadian, metabolic, and gene programming interplay during cell maturation?
How do maturing cells sense and adapt to external electromechanical forces?
How does crosstalk between tissues promote maturational change?
How are electromechanical stimuli transduced to program maturational adaptations?
What about the in vivo milieu promotes maturity?
ACKNOWLEDGEMENTS
We thank Harvey Lodish, Katie Villa, Jose Rivera-Feliciano, and members of our laboratories for helpful discussions and feedback on this manuscript, and Tom DiCesare for help with the illustrations. This work was supported by the Howard Hughes Medical Institute and by grants from the NIH (1K01DK129442-01) and Human Islet Research Network (U24DK104162-07) to J.R.A-D. and from the Harvard Stem Cell Institute, Helmsley Charitable Trust, Juvenile Diabetes Research Foundation, and the JPB Foundation to D.A.M. We apologize to colleagues whose work could not be referenced or discussed due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
D.A.M. is a founder of Semma Therapeutics and consultant for Semma Therapeutics/Vertex.
J.R.A.-D. declares no competing interests.
REFERENCES
- Abbott LF, and Nelson SB (2000). Synaptic plasticity: taming the beast. Nat Neurosci 3 Suppl, 1178–1183. [DOI] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C, DiIenno A, Hollister-Lock J, Cahill C, Sharma A, Weir G, Colton C, and Bonner-Weir S (2015). MAFA and T3 Drive Maturation of Both Fetal Human Islets and Insulin-Producing Cells Differentiated From hESC. J Clin Endocrinol Metab 100, 3651–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C, Koh A, El Khattabi I, Li WC, Toschi E, Jermendy A, Juhl K, Mao K, Weir GC, Sharma A, et al. (2011). Mafa expression enhances glucose-responsive insulin secretion in neonatal rat beta cells. Diabetologia 54, 583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo-Mazzucato C, Zavacki AM, Marinelarena A, Hollister-Lock J, El Khattabi I, Marsili A, Weir GC, Sharma A, Larsen PR, and Bonner-Weir S (2013). Thyroid hormone promotes postnatal rat pancreatic beta-cell development and glucose-responsive insulin secretion through MAFA. Diabetes 62, 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Dominguez JR, Donaghey J, Rasouli N, Kenty JHR, Helman A, Charlton J, Straubhaar JR, Meissner A, and Melton DA (2020). Circadian Entrainment Triggers Maturation of Human In Vitro Islets. Cell Stem Cell 26, 108–122 e110. [DOI] [PubMed] [Google Scholar]
- An X, and Mohandas N (2008). Disorders of red cell membrane. Br J Haematol 141, 367–375. [DOI] [PubMed] [Google Scholar]
- Artner I, Hang Y, Mazur M, Yamamoto T, Guo M, Lindner J, Magnuson MA, and Stein R (2010). MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 59, 2530–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avior Y, Levy G, Zimerman M, Kitsberg D, Schwartz R, Sadeh R, Moussaieff A, Cohen M, Itskovitz-Eldor J, and Nahmias Y (2015). Microbial-derived lithocholic acid and vitamin K2 drive the metabolic maturation of pluripotent stem cells-derived and fetal hepatocytes. Hepatology 62, 265–278. [DOI] [PubMed] [Google Scholar]
- Baeyens L, Hindi S, Sorenson RL, and German MS (2016). beta-Cell adaptation in pregnancy. Diabetes Obes Metab 18 Suppl 1, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass J, and Lazar MA (2016). Circadian time signatures of fitness and disease. Science 354, 994–999. [DOI] [PubMed] [Google Scholar]
- Bass J, and Takahashi JS (2010). Circadian integration of metabolism and energetics. Science 330, 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, and Canbay A (2012). The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol 56, 952–964. [DOI] [PubMed] [Google Scholar]
- Bedont JL, Iascone DM, and Sehgal A (2020). The Lineage Before Time: Circadian and Nonclassical Clock Influences on Development. Annu Rev Cell Dev Biol 36, 469–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger DR, Ware BR, Davidson MD, Allsup SR, and Khetani SR (2015). Enhancing the functional maturity of induced pluripotent stem cell-derived human hepatocytes by controlled presentation of cell-cell interactions in vitro. Hepatology 61, 1370–1381. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, et al. (2009). Evidence for cardiomyocyte renewal in humans. Science 324, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers DM (2002). Cardiac excitation-contraction coupling. Nature 415, 198–205. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, and Guillemot F (2002). Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3, 517–530. [DOI] [PubMed] [Google Scholar]
- Bertrand S, and Cazalets JR (1998). Postinhibitory rebound during locomotor-like activity in neonatal rat motoneurons in vitro. J Neurophysiol 79, 342–351. [DOI] [PubMed] [Google Scholar]
- Bertrand SS, and Cazalets JR (2013). Activity-dependent synaptic plasticity and metaplasticity in spinal motor networks. Curr Pharm Des 19, 4498–4508. [DOI] [PubMed] [Google Scholar]
- Bideyan L, Nagari R, and Tontonoz P (2021). Hepatic transcriptional responses to fasting and feeding. Genes Dev 35, 635–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MD, Rexius-Hall ML, Elgass LJ, and Eddington DT (2014). Oxygen control with microfluidics. Lab Chip 14, 4305–4318. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, and Butler PC (2003). Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, and Brummelkamp TR (2007). YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17, 2054–2060. [DOI] [PubMed] [Google Scholar]
- Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, and Thornburg KL (2012). Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J 26, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, and Shenoy VB (2020). Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho CS, Yoon HJ, Kim JY, Woo HA, and Rhee SG (2014). Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc Natl Acad Sci U S A 111, 12043–12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DC, Koo Y, Xu K, Fu S, and Cleaver O (2011). Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn 240, 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia C, Koshkin A, Duarte P, Hu D, Teixeira A, Domian I, Serra M, and Alves PM (2017). Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci Rep 7, 8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, and Schibler U (2000). Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14, 2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M, and Mooney DJ (2017). Leveraging advances in biology to design biomaterials. Nat Mater 16, 1178–1185. [DOI] [PubMed] [Google Scholar]
- Davis-Dusenbery BN, Williams LA, Klim JR, and Eggan K (2014). How to make spinal motor neurons. Development 141, 491–501. [DOI] [PubMed] [Google Scholar]
- Delaunay J (2007). The molecular basis of hereditary red cell membrane disorders. Blood Rev 21, 1–20. [DOI] [PubMed] [Google Scholar]
- Discher DE (2000). New insights into erythrocyte membrane organization and microelasticity. Curr Opin Hematol 7, 117–122. [DOI] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, and Melton DA (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46. [DOI] [PubMed] [Google Scholar]
- Duan X, Fu TM, Liu J, and Lieber CM (2013). Nanoelectronics-biology frontier: From nanoscopic probes for action potential recording in live cells to three-dimensional cyborg tissues. Nano Today 8, 351–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn KK, Reichardt IM, Simmons AD, Jin G, Floy ME, Hoon KM, and Palecek SP (2019). Coculture of Endothelial Cells with Human Pluripotent Stem Cell-Derived Cardiac Progenitors Reveals a Differentiation Stage-Specific Enhancement of Cardiomyocyte Maturation. Biotechnol J 14, e1800725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir T, Timko BP, Kohane DS, and Langer R (2011). Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol 6, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgsaeter A, Stokke BT, Mikkelsen A, and Branton D (1986). The molecular basis of erythrocyte shape. Science 234, 1217–1223. [DOI] [PubMed] [Google Scholar]
- Eng G, Lee BW, Protas L, Gagliardi M, Brown K, Kass RS, Keller G, Robinson RB, and Vunjak-Novakovic G (2016). Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat Commun 7, 10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, and Discher DE (2006). Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689. [DOI] [PubMed] [Google Scholar]
- Esch MB, King TL, and Shuler ML (2011). The role of body-on-a-chip devices in drug and toxicity studies. Annu Rev Biomed Eng 13, 55–72. [DOI] [PubMed] [Google Scholar]
- Fajans SS, Bell GI, and Polonsky KS (2001). Molecular mechanisms and clinical pathophysiology of maturity-onset diabetes of the young. N Engl J Med 345, 971–980. [DOI] [PubMed] [Google Scholar]
- Feaster TK, Cadar AG, Wang L, Williams CH, Chun YW, Hempel JE, Bloodworth N, Merryman WD, Lim CC, Wu JC, et al. (2015). Matrigel Mattress: A Method for the Generation of Single Contracting Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res 117, 995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiner R, and Dvir T (2017). Tissue–electronics interfaces: from implantable devices to engineered tissues. Nature Reviews Materials 3, 17076. [Google Scholar]
- Feridooni HA, Dibb KM, and Howlett SE (2015). How cardiomyocyte excitation, calcium release and contraction become altered with age. J Mol Cell Cardiol 83, 62–72. [DOI] [PubMed] [Google Scholar]
- Friedman CE, Nguyen Q, Lukowski SW, Heifer A, Chiu HS, Miklas J, Levy S, Suo S, Han JJ, Osteil P, et al. (2018). Single-Cell Transcriptomic Analysis of Cardiac Differentiation from Human PSCs Reveals HOPX-Dependent Cardiomyocyte Maturation. Cell Stem Cell 23, 586–598 e588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbern JC, Helman A, Sereda R, Sarikhani M, Ahmed A, Escalante GO, Ogurlu R, Kim SL, Zimmerman JF, Cho A, et al. (2020). Inhibition of mTOR Signaling Enhances Maturation of Cardiomyocytes Derived From Human-Induced Pluripotent Stem Cells via p53-Induced Quiescence. Circulation 141, 285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P (2018). A Review of Podocyte Biology. Am J Nephrol 47 Suppl 1, 3–13. [DOI] [PubMed] [Google Scholar]
- Gentillon C, Li D, Duan M, Yu WM, Preininger MK, Jha R, Rampoldi A, Saraf A, Gibson GC, Qu CK, et al. (2019). Targeting HIF-1alpha in combination with PPARalpha activation and postnatal factors promotes the metabolic maturation of human induced pluripotent stem cell-derived cardiomyocytes. J Mol Cell Cardiol 132, 120–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granit R, Kernell D, and Shortess GK (1963). Quantitative Aspects of Repetitive Firing of Mammalian Motoneurones, Caused by Injected Currents. J Physiol 168, 911–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S (2003). The motor infrastructure: from ion channels to neuronal networks. Nat Rev Neurosci 4, 573–586. [DOI] [PubMed] [Google Scholar]
- Guo R, Xu X, Lu Y, and Xie X (2017). Physiological oxygen tension reduces hepatocyte dedifferentiation in in vitro culture. Sci Rep 7, 5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang W, He B, Chen J, Xia L, Wen B, Liang T, Wang X, Zhang Q, Wu Y, Chen Q, et al. (2018). Berberine Ameliorates High Glucose-Induced Cardiomyocyte Injury via AMPK Signaling Activation to Stimulate Mitochondrial Biogenesis and Restore Autophagic Flux. Front Pharmacol 9, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattersley AT, Greeley SAW, Polak M, Rubio-Cabezas O, Njolstad PR, Mlynarski W, Castano L, Carlsson A, Raile K, Chi DV, et al. (2018). ISPAD Clinical Practice Consensus Guidelines 2018: The diagnosis and management of monogenic diabetes in children and adolescents. Pediatr Diabetes 19 Suppl 27, 47–63. [DOI] [PubMed] [Google Scholar]
- Helman A, Cangelosi AL, Davis JC, Pham Q, Rothman A, Faust AL, Straubhaar JR, Sabatini DM, and Melton DA (2020). A Nutrient-Sensing Transition at Birth Triggers Glucose-Responsive Insulin Secretion. Cell Metab 31, 1004–1016 e1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henslee EA, Crosby P, Kitcatt SJ, Parry JSW, Bernardini A, Abdallat RG, Braun G, Fatoyinbo HO, Harrison EJ, Edgar RS, et al. (2017). Rhythmic potassium transport regulates the circadian clock in human red blood cells. Nat Commun 8, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrup K, and Yang Y (2007). Cell cycle regulation in the postmitotic neuron: oxymoron or new biology? Nat Rev Neurosci 8, 368–378. [DOI] [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, and Ziskind-Conhaim L (2005). Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol 93, 1439–1449. [DOI] [PubMed] [Google Scholar]
- Hofer M, and Lutolf MP (2021). Engineering organoids. Nat Rev Mater, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, et al. (2019). Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvatin S, O'Donnell CW, Deng F, Millman JR, Pagliuca FW, DiIorio P, Rezania A, Gifford DK, and Melton DA (2014). Differentiated human stem cells resemble fetal, not adult, beta cells. Proc Natl Acad Sci U S A 111, 3038–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Linders A, Yamak A, Correia C, Kijlstra JD, Garakani A, Xiao L, Milan DJ, van der Meer P, Serra M, et al. (2018). Metabolic Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes by Inhibition of HIF1alpha and LDHA. Circ Res 123, 1066–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Walker EM, Dadi PK, Hu R, Xu Y, Zhang W, Sanavia T, Mun J, Liu J, Nair GG, et al. (2018). Synaptotagmin 4 Regulates Pancreatic beta Cell Maturation by Modulating the Ca(2+) Sensitivity of Insulin Secretion Vesicles. Dev Cell 45, 347–361 e345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh D, Hamilton GA, and Ingber DE (2011). From 3D cell culture to organs-on-chips. Trends Cell Biol 21, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Dufresne ER, and Schwartz MA (2014). Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15, 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Kakuta S, Kawasaki Y, Miyaki T, Nonami T, Miyazaki N, Nakao T, Enomoto S, Arai S, Koike M, et al. (2017). Morphological process of podocyte development revealed by block-face scanning electron microscopy. J Cell Sci 130, 132–142. [DOI] [PubMed] [Google Scholar]
- Jaafar R, Tran S, Shah AN, Sun G, Valdearcos M, Marchetti P, Masini M, Swisa A, Giacometti S, Bernal-Mizrachi E, et al. (2019). mTORC1 to AMPK switching underlies beta-cell metabolic plasticity during maturation and diabetes. J Clin Invest 129, 4124–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacovetti C, Matkovich SJ, Rodriguez-Trejo A, Guay C, and Regazzi R (2015). Postnatal beta-cell maturation is associated with islet-specific microRNA changes induced by nutrient shifts at weaning. Nat Commun 6, 8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakesova M, Silvera Ejneby M, Derek V, Schmidt T, Gryszel M, Brask J, Schindl R, Simon DT, Berggren M, Elinder F, et al. (2019). Optoelectronic control of single cells using organic photocapacitors. Sci Adv 5, eaav5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya A, Kinoshita T, and Miyajima A (2001). Oncostatin M and hepatocyte growth factor induce hepatic maturation via distinct signaling pathways. FEBS Lett 492, 90–94. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Kaplan A, and Henderson CE (2010). Motor neuron diversity in development and disease. Annu Rev Neurosci 33, 409–440. [DOI] [PubMed] [Google Scholar]
- Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, and Murry CE (2020). Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol 17, 341–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K, Han SI, Shioda S, Hirai M, Nishizawa M, and Handa H (2002). MafA is a glucose-regulated and pancreatic beta-cell-specific transcriptional activator for the insulin gene. J Biol Chem 277, 49903–49910. [DOI] [PubMed] [Google Scholar]
- Katz LC, and Shatz CJ (1996). Synaptic activity and the construction of cortical circuits. Science 274, 1133–1138. [DOI] [PubMed] [Google Scholar]
- Kidambi S, Yarmush RS, Novik E, Chao P, Yarmush ML, and Nahmias Y (2009). Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. Proc Natl Acad Sci U S A 106, 15714–15719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Lipke EA, Kim P, Cheong R, Thompson S, Delannoy M, Suh KY, Tung L, and Levchenko A (2010). Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Proc Natl Acad Sci U S A 107, 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkby LA, Sack GS, Firl A, and Feller MB (2013). A role for correlated spontaneous activity in the assembly of neural circuits. Neuron 80, 1129–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima N (2014). In vitro reconstitution of pancreatic islets. Organogenesis 10, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook H, Lepore JJ, Gitler AD, Lu MM, Wing-Man Yung W, Mackay J, Zhou R, Ferrari V, Gruber P, and Epstein JA (2003). Cardiac hypertrophy and histone deacetylase-dependent transcriptional repression mediated by the atypical homeodomain protein Hop. J Clin Invest 112, 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn V, Diederich L, Keller T.C.S.t., Kramer CM, Luckstadt W, Panknin C, Suvorava T, Isakson BE, Kelm M, and Cortese-Krott MM (2017). Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid Redox Signal 26, 718–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, and Murry CE (2011). Heart regeneration. Nature 473, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju A, Umapathy A, Tan LX, Daniele L, Philp NJ, Boesze-Battaglia K, and Williams DS (2020). The cell biology of the retinal pigment epithelium. Prog Retin Eye Res, 100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, and Knoblich JA (2014). Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125. [DOI] [PubMed] [Google Scholar]
- Larsen FL, Katz S, Roufogalis BD, and Brooks DE (1981). Physiological shear stresses enhance the Ca2+ permeability of human erythrocytes. Nature 294, 667–668. [DOI] [PubMed] [Google Scholar]
- Lee DS, Chen JH, Lundy DJ, Liu CH, Hwang SM, Pabon L, Shieh RC, Chen CC, Wu SN, Yan YT, et al. (2015). Defined MicroRNAs Induce Aspects of Maturation in Mouse and Human Embryonic-Stem-Cell-Derived Cardiomyocytes. Cell Rep 12, 1960–1967. [DOI] [PubMed] [Google Scholar]
- Lee HP, Gu L, Mooney DJ, Levenston ME, and Chaudhuri O (2017). Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat Mater 16, 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YK, Ng KM, Chan YC, Lai WH, Au KW, Ho CY, Wong LY, Lau CP, Tse HF, and Siu CW (2010). Triiodothyronine promotes cardiac differentiation and maturation of embryonic stem cells via the classical genomic pathway. Mol Endocrinol 24, 1728–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik L, Zhihua H, Mobini S, Thottakkattumana Parameswaran V, Eischen-Loges M, Slavici A, Helbing J, Pindur L, Oliveira KMC, Bhavsar MB, et al. (2018). Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci Rep 8, 6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nan K, Le Floch P, Lin Z, Sheng H, Blum TS, and Liu J (2019). Cyborg Organoids: Implantation of Nanoelectronics via Organogenesis for Tissue-Wide Electrophysiology. Nano Lett 19, 5781–5789. [DOI] [PubMed] [Google Scholar]
- Little MH, and Combes AN (2019). Kidney organoids: accurate models or fortunate accidents. Genes Dev 33, 1319–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Fu TM, Cheng Z, Hong G, Zhou T, Jin L, Duvvuri M, Jiang Z, Kruskal P, Xie C, et al. (2015). Syringe-injectable electronics. Nat Nanotechnol 10, 629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, et al. (2018). Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 36, 597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marian AJ, and Braunwald E (2017). Hypertrophic Cardiomyopathy: Genetics, Pathogenesis, Clinical Manifestations, Diagnosis, and Therapy. Circ Res 121, 749–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlier Q, D'Aes T, Verteneuil S, Vandenbosch R, and Malgrange B (2020). Core cell cycle machinery is crucially involved in both life and death of post-mitotic neurons. Cell Mol Life Sci 77, 4553–4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martewicz S, Serena E, Zatti S, Keller G, and Elvassore N (2017). Substrate and mechanotransduction influence SERCA2a localization in human pluripotent stem cell-derived cardiomyocytes affecting functional performance. Stem Cell Res 25, 107–114. [DOI] [PubMed] [Google Scholar]
- May-Simera HL, Wan Q, Jha BS, Hartford J, Khristov V, Dejene R, Chang J, Patnaik S, Lu Q, Banerjee P, et al. (2018). Primary Cilium-Mediated Retinal Pigment Epithelium Maturation Is Disrupted in Ciliopathy Patient Cells. Cell Rep 22, 189–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain ML, and Parker KK (2011). Mechanotransduction: the role of mechanical stress, myocyte shape, and cytoskeletal architecture on cardiac function. Pflugers Arch 462, 89–104. [DOI] [PubMed] [Google Scholar]
- Meehan CF, Sukiasyan N, Zhang M, Nielsen JB, and Hultborn H (2010). Intrinsic properties of mouse lumbar motoneurons revealed by intracellular recording in vivo. J Neurophysiol 103, 2599–2610. [DOI] [PubMed] [Google Scholar]
- Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, et al. (2017). Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci U S A 114, E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, and Srivastava D (2018). Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 173, 104–116 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas N, and Gallagher PG (2008). Red cell membrane: past, present, and future. Blood 112, 3939–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty JG, Nagababu E, and Rifkind JM (2014). Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front Physiol 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni L, Burdick JA, Highley C, Lee SJ, Morimoto Y, Takeuchi S, and Yoo JJ (2018). Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat Rev Mater 3, 21–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola JP, and Seifert EL (2002). Circadian patterns of breathing. Respir Physiol Neurobiol 131, 91–100. [DOI] [PubMed] [Google Scholar]
- Nakano H, Minami I, Braas D, Pappoe H, Wu X, Sagadevan A, Vergnes L, Fu K, Morselli M, Dunham C, et al. (2017). Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, and Jessell TM (2001). Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron 31, 773–789. [DOI] [PubMed] [Google Scholar]
- Noy A (2011). Bionanoelectronics. Adv Mater 23, 807–820. [DOI] [PubMed] [Google Scholar]
- Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, et al. (2013). Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 10, 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill JS, and Reddy AB (2011). Circadian clocks in human red blood cells. Nature 469, 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrot M, Rud J, Moss LG, and Sharma A (2002). Identification of beta-cell-specific insulin gene transcription factor RIPE3b1 as mammalian MafA. Proc Natl Acad Sci U S A 99, 6737–6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S (2016). Circadian physiology of metabolism. Science 354, 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SS, Blackwell DJ, Gomez-Hurtado N, Frisk M, Wang L, Kim K, Dahl CP, Fiane A, Tonnessen T, Kryshtal DO, et al. (2017). Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ Res 121, 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipatpolkai T, Usher S, Stansfeld PJ, and Ashcroft FM (2020). New insights into KATP channel gene mutations and neonatal diabetes mellitus. Nat Rev Endocrinol 16, 378–393. [DOI] [PubMed] [Google Scholar]
- Politis PK, Thomaidou D, and Matsas R (2008). Coordination of cell cycle exit and differentiation of neuronal progenitors. Cell Cycle 7, 691–697. [DOI] [PubMed] [Google Scholar]
- Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, et al. (2011). Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab 13, 440–449. [DOI] [PubMed] [Google Scholar]
- Prentki M, Matschinsky FM, and Madiraju SR (2013). Metabolic signaling in fuel-induced insulin secretion. Cell Metab 18, 162–185. [DOI] [PubMed] [Google Scholar]
- Puente BN, Kimura W, Muralidhar SA, Moon J, Amatruda JF, Phelps KL, Grinsfelder D, Rothermel BA, Chen R, Garcia JA, et al. (2014). The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 157, 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, and Vunjak-Novakovic G (2004). Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A 101, 18129–18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao C, Prodromakis T, Kolker L, Chaudhry UA, Trantidou T, Sridhar A, Weekes C, Camelliti P, Harding SE, Darzi A, et al. (2013). The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 34, 2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, and Feldman JL (2000). Synaptic control of motoneuronal excitability. Physiol Rev 80, 767–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoux C, Faivre M, Bessaa A, Da Costa L, Joly P, Gauthier A, and Connes P (2019). Impact of surface-area-to-volume ratio, internal viscosity and membrane viscoelasticity on red blood cell deformability measured in isotonic condition. Sci Rep 9, 6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, and Pruitt BL (2015). Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A 112, 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolo LJ, Peng S, Luo Y, and Xiao W (2011). Integration of tight junctions and claudins with the barrier functions of the retinal pigment epithelium. Prog Retin Eye Res 30, 296–323. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Sahoo JK, McNamara LE, Burgess KV, Yang J, Alakpa EV, Anderson HJ, Hay J, Turner LA, Yarwood SJ, et al. (2016). Dynamic Surfaces for the Study of Mesenchymal Stem Cell Growth through Adhesion Regulation. ACS Nano 10, 6667–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rog-Zielinska EA, Craig MA, Manning JR, Richardson RV, Gowans GJ, Dunbar DR, Gharbi K, Kenyon CJ, Holmes MC, Hardie DG, et al. (2015). Glucocorticoids promote structural and functional maturation of foetal cardiomyocytes: a role for PGC-1alpha. Cell Death Differ 22, 1106–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, and Vunjak-Novakovic G (2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorsman P, and Ashcroft FM (2018). Pancreatic beta-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol Rev 98, 117–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, and Murry CE (2016). Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue. Circulation 134, 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Cabezas O, and Ellard S (2013). Diabetes mellitus in neonates and infants: genetic heterogeneity, clinical approach to diagnosis, and therapeutic options. Horm Res Paediatr 80, 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J, Reick M, and McKnight SL (2002). Metabolism and the control of circadian rhythms. Annu Rev Biochem 71, 307–331. [DOI] [PubMed] [Google Scholar]
- Sarikhani M, Garbern JC, Ma S, Sereda R, Conde J, Krahenbuhl G, Escalante GO, Ahmed A, Buenrostro JD, and Lee RT (2020). Sustained Activation of AMPK Enhances Differentiation of Human iPSC-Derived Cardiomyocytes via Sirtuin Activation. Stem Cell Reports 15, 498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y (2013). Cytosystems dynamics in self-organization of tissue architecture. Nature 493, 318–326. [DOI] [PubMed] [Google Scholar]
- Scott RP, and Quaggin SE (2015). Review series: The cell biology of renal filtration. J Cell Biol 209, 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Sances S, Workman MJ, and Svendsen CN (2020). Multi-lineage Human iPSC-Derived Platforms for Disease Modeling and Drug Discovery. Cell Stem Cell 26, 309–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheydina A, Riordon DR, and Boheler KR (2011). Molecular mechanisms of cardiomyocyte aging. Clin Sci (Lond) 121, 315–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, and Monga SP (2013). Cellular and molecular basis of liver development. Compr Physiol 3, 799–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, and Duncan SA (2010). Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology 51, 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedner S, Kruger M, Schroeter M, Metzler D, Roell W, Fleischmann BK, Hescheler J, Pfitzer G, and Stehle R (2003). Developmental changes in contractility and sarcomeric proteins from the early embryonic to the adult stage in the mouse heart. J Physiol 548, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinturel F, Gos P, Petrenko V, Hagedorn C, Kreppel F, Storch KF, Knutti D, Liani A, Weitz C, Emmenegger Y, et al. (2021). Circadian hepatocyte clocks keep synchrony in the absence of a master pacemaker in the suprachiasmatic nucleus or other extrahepatic clocks. Genes Dev 35, 329–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Q, Macklin B, Chan XY, Jones H, Trempel M, Yoder MC, and Gerecht S (2018). Differential HDAC6 Activity Modulates Ciliogenesis and Subsequent Mechanosensing of Endothelial Cells Derived from Pluripotent Stem Cells. Cell Rep 24, 895–908 e896. [DOI] [PubMed] [Google Scholar]
- Spach MS, Heidlage JF, Barr RC, and Dolber PC (2004). Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm 1, 500–515. [DOI] [PubMed] [Google Scholar]
- Sparrow JR, Hicks D, and Hamel CP (2010). The retinal pigment epithelium in health and disease. Curr Mol Med 10, 802–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, and Menaker M (2001). Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493. [DOI] [PubMed] [Google Scholar]
- Stolovich-Rain M, Enk J, Vikesa J, Nielsen FC, Saada A, Glaser B, and Dor Y (2015). Weaning triggers a maturation step of pancreatic beta cells. Dev Cell 32, 535–545. [DOI] [PubMed] [Google Scholar]
- Storm T, Burgoyne T, and Futter CE (2020). Membrane trafficking in the retinal pigment epithelium at a glance. J Cell Sci 133. [DOI] [PubMed] [Google Scholar]
- Strauss O (2005). The retinal pigment epithelium in visual function. Physiol Rev 85, 845–881. [DOI] [PubMed] [Google Scholar]
- Stuck BJ, Lenski M, Bohm M, and Laufs U (2008). Metabolic switch and hypertrophy of cardiomyocytes following treatment with angiotensin II are prevented by AMP-activated protein kinase. J Biol Chem 283, 32562–32569. [DOI] [PubMed] [Google Scholar]
- Takano AP, Diniz GP, and Barreto-Chaves ML (2013). AMPK signaling pathway is rapidly activated by T3 and regulates the cardiomyocyte growth. Mol Cell Endocrinol 376, 43–50. [DOI] [PubMed] [Google Scholar]
- Tian B, and Lieber CM (2019). Nanowired Bioelectric Interfaces. Chem Rev 119, 9136–9152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian B, Liu J, Dvir T, Jin L, Tsui JH, Qing Q, Suo Z, Langer R, Kohane DS, and Lieber CM (2012). Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat Mater 11, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilles AW, Baskaran H, Roy P, Yarmush ML, and Toner M (2001). Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol Bioeng 73, 379–389. [DOI] [PubMed] [Google Scholar]
- Trefts E, Gannon M, and Wasserman DH (2017). The liver. Curr Biol 27, R1147–R1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegas AJ, Veiseh O, Gurtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, et al. (2016). Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice. Nat Med 22, 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco S, Paulsen B, and Arlotta P (2020). 3D Brain Organoids: Studying Brain Development and Disease Outside the Embryo. Annu Rev Neurosci 43, 375–389. [DOI] [PubMed] [Google Scholar]
- Velazco-Cruz L, Song J, Maxwell KG, Goedegebuure MM, Augsornworawat P, Hogrebe NJ, and Millman JR (2019). Acquisition of Dynamic Function in Human Stem Cell-Derived beta Cells. Stem Cell Reports 12, 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JH, Harb G, Poh YC, Sintov E, Gurtler M, Pagliuca FW, et al. (2019). Charting cellular identity during human in vitro beta-cell differentiation. Nature 569, 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Mark K, Gauss V, von der Mark H, and Muller P (1977). Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267, 531–532. [DOI] [PubMed] [Google Scholar]
- Wang D, Wang J, Bai L, Pan H, Feng H, Clevers H, and Zeng YA (2020). Long-Term Expansion of Pancreatic Islet Organoids from Resident Procr(+) Progenitors. Cell 180, 1198–1211 e1119. [DOI] [PubMed] [Google Scholar]
- Wang H, Brun T, Kataoka K, Sharma AJ, and Wollheim CB (2007). MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia 50, 348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir GC, Laybutt DR, Kaneto H, Bonner-Weir S, and Sharma A (2001). Beta-cell adaptation and decompensation during the progression of diabetes. Diabetes 50 Suppl 1, S154–159. [DOI] [PubMed] [Google Scholar]
- Werley CA, Chien MP, Gaublomme J, Shekhar K, Butty V, Yi BA, Kralj JM, Bloxham W, Boyer LA, Regev A, et al. (2017). Geometry-dependent functional changes in iPSC-derived cardiomyocytes probed by functional imaging and RNA sequencing. PLoS One 12, e0172671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, O'Cearbhaill ED, Liu SS, Zhou A, Chitnis GD, Hamilos AE, Xu J, Verma MKS, Giraldo JA, Kudo Y, et al. (2021). A therapeutic convection-enhanced macroencapsulation device for enhancing beta cell viability and insulin secretion. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara E, Wei Z, Lin CS, Fang S, Ahmadian M, Kida Y, Tseng T, Dai Y, Yu RT, Liddle C, et al. (2016). ERRgamma Is Required for the Metabolic Maturation of Therapeutically Functional Glucose-Responsive beta Cells. Cell Metab 23, 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, et al. (2005). MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol 25, 4969–4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Rezvani M, Harbell J, Mattis AN, Wolfe AR, Benet LZ, Willenbring H, and Ding S (2014). Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature 508, 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman AP, Gomez-Viquez NL, Bloch RJ, and Lederer WJ (2010). Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol 48, 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]