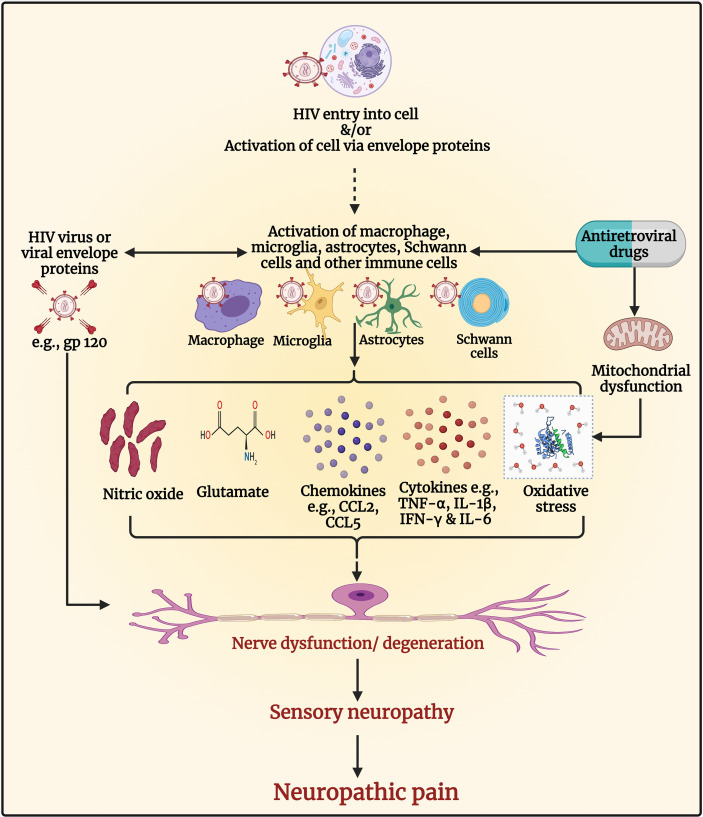
Figure 4.
Pathogenesis of human immunodeficiency virus (HIV) associated sensory neuropathy and neuropathic pain. The entry of HIV in macrophages or microglia results in their activation and the release of proinflammatory cytokines, chemokines, glutamate, nitric oxide, and viral envelope proteins, including glycoprotein (gp)120, which can cause nerve dysfunction/neurodegeneration. The viral envelope gp120 has a direct neuropathic effect on neurons due to activation of chemokine receptors resulting in neuronal hyperexcitability and neuropathic pain. It also has indirect neuropathic effects through the activation of macrophages and Schwann cells. The presence of proinflammatory cytokines within the peripheral nerve and dorsal root ganglia causes infiltration of macrophages and lymphocytes, which secrete inflammatory cytokines (e.g., tumor necrosis factor-alpha [TNF-α], interleukin-1 beta [IL-1β], interferon-gamma [IFN-γ], and IL-6), and chemokines (e.g., C-C motif ligand 2 [CCL2] and CCL5) and exacerbate nerve degeneration leading to neuropathy and neuropathic pain. Antiretroviral drugs such as nucleoside reverse transcriptase inhibitors (NRTIs) inhibit deoxyribonucleic acid (DNA) γ- polymerase, the enzyme essential for copying and repair of mitochondrial DNA. This results in the accumulation of mutations of mitochondrial DNA, defective respiratory chain subunits, impaired oxidative phosphorylation, reduced adenosine triphosphate (ATP), and oxidative stress. Oxidative stress causes nerve degeneration. The NRTIs also contribute to neuropathy and neuropathic pain by activating glial and immune cells to release cytokines, chemokines, and molecules that induce neuronal hyperexcitability and neurodegeneration. Figure created by EA and WM using BioRender.com.