Summary
Here we describe an optimized protocol for X-gal staining of tissue clearing embryo and adult mouse using CUBIC. The activity of LacZ knock-in reflecting endogenous expression of genes of interest in the whole body can be visualized by X-gal staining. This protocol is suitable for examining the developmental stage-specific expression of genes of interest spatially and temporally.
For complete details on the use and execution of this protocol, please refer to Watanabe-Takano et al. (2021).
Subject areas: Developmental biology, Genetics, Microbiology, Model Organisms
Graphical abstract
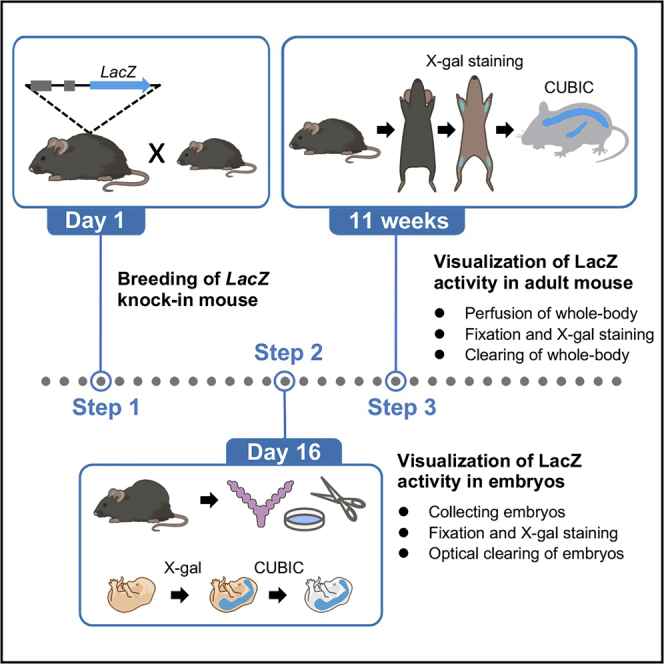
Highlights
-
•
Breeding of LacZ knock-in mouse
-
•
Protocol for the fixation and perfusion for embryo and adult mouse
-
•
Protocol for the visualization of LacZ activity in embryo and adult mouse
-
•
Clearing method for the embryo and mice after color developing
Here we describe an optimized protocol for X-gal staining of tissue clearing embryo and adult mouse using CUBIC. The activity of LacZ knock-in reflecting endogenous expression of genes of interest in the whole body can be visualized by X-gal staining. This protocol is suitable for examining the developmental stage-specific expression of genes of interest spatially and temporally.
Before you begin
Breeding mice
Timing: 16 days for E15.5 embryos, 11 weeks for adult mice
-
1.
Prepare mutant mice expressing LacZ and breed them with wild-type or mutants.
Note: You will get higher signals in homozygotes as compared to that in heterozygotes. However, you need to keep in mind that homozygotes will cause early death or tissue defects due to the gene deletion using reporter targeting methods.
-
2.
For an embryonic assessment, prepare a pregnant mouse by checking a vaginal plug on the next morning of mating. The vaginal plug marks the embryonic day (E) 0.5. Embryos will be taken at E15.5.
Note: Plan the dissection timing by considering the appropriate timing when the lacZ expression should be examined. When planning the dissection date, the plugs might be the hint for the pregnancy, although they are not always reliable.
You need an appropriate number of pregnant mice for analyses, because the presence or absence of plugs does not always reflect pregnancy.
-
3.
Regarding an expression analysis in adult mouse, grow the progenies until they become 8 weeks.
Note: Check the insertion of LacZ as well as the gene of your interest by genotyping when LacZ is knocked-in the certain locus of the gene of your interest before the dissection and prepare wild-type (siblings) as a negative control.
Key resources table
| REAGENTS | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 4% Paraformaldehyde phosphate buffer solution | FUJIFILM | 163-20145 CAS:30525-89-4 |
| 5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside | FUJIFILM | 027-07854 CAS:7240-90-6 |
| 25% Glutaraldehyde | Nacalai Tesque | 17003-92 CAS:111-30-8 |
| Butorphanol | Meiji Seika | CAS:42408-82-2 |
| K-ferricyanide | FUJIFILM | 152559 CAS:13746-66-2 |
| K-ferrocyanide | FUJIFILM | 152560 CAS:14459-95-1 |
| Medetomidine | Orion corporation | CAS:86347-14-0 |
| MgCl2 | FUJIFILM | 136-03995 CAS:7786-30-3 |
| Midazolam | SANDOZ | CAS:59467-70-8 |
| N,N-Dimethylformamide (DMFA) | FUJIFILM | 045-02916 CAS:68-12-2 |
| N,N,N′,N′-Tetrakis (2-hydroxypropyl) ethylenediamine | Tokyo Chemical Industry, Co | T0781 CAS:102-60-3 |
| NaCl | Nacalai Tesque | 31319-45 CAS:7647-14-5 |
| NaH2PO42H2O | FUJIFILM | 199-02825 CAS: 13472-35-0 |
| Na2HPO412H2O | FUJIFILM | 196-02835 CAS:10039-32-4 |
| NaOH | Nacalai Tesque | 31511-05 CAS: 1310-73-2 |
| NP-40 substitute | FUJIFILM | 145-09701 CAS:9016-45-9 |
| O,O′-Bis (2-aminoethyl) ethyleneglycol-N,N,N′,N′-tetraacetic acid (EGTA) | Dojindo | 348-01311 CAS:67-42-5 |
| Polyoxyethylene(10) Octylphenyl Ether (TritonX-100) | FUJIFILM | 162-24755 CAS:9002-93-1 |
| Saline | Otsuka Pharmaceutical Factory | N/A |
| Sodium dodecyl sulfate | SERVA | 20765.02 CAS: 151-21-3 |
| Trizma base | Sigma-Aldrich | T1503 CAS:77-86-1 |
| Urea | Wako | 211-01213 CAS:57-13-6 |
| Experimental models: Organisms/strains | ||
| B6.129S-OSTNtm1a(KOMP)ncvc | Laboratory of Naoki Mochizuki | N/A |
| Instruments or equipment | ||
| Clear plastic case | TAKACHI ELECTRONICS ENCLOSURE Co., Ltd. | PB18-3-8 |
| Digital camera | Olympus | DP21 |
| Electric clipper | Thrive | Model 808 |
| Heat sealer | FUJI IMPULSE | POLYSEALER P200 |
| In vitro shaker | TAITEC Corporation | Wave-SI |
| Light box | FUJICOLOR | New 5000 inverter |
| Needle | TERUMO | NN-2516R |
| Perista pump | ATTO | AC-2110 II |
| Polyethylene bag | TAIYO | N/A |
| Stereomicroscope | Olympus | SZX7 |
| T shape stopcock | TERMO | TS-TR1K |
| Winged needles | TERMO | SV-23DLK |
Materials and equipment
1×PBS
| Reagent | Final concentration | Amount |
|---|---|---|
| NaH2PO42H2O | 15 mM | 46.8 g |
| NaCl | 145.5 mM | 170 g |
| NaOH | n/a | 10 g |
| Total | n/a | 20 L |
Dissolve with 5 L ddH2O, adjust pH to 7.2, and then add ddH2O up to 20 L.
Store at room temperature.
1×PBS containing Mg2+
| Reagent | Final concentration | Amount |
|---|---|---|
| 1×PBS | n/a | 1L |
| 1 M MgCl2 | 2 mM | 2 mL |
| Total | n/a | 1 L |
Store at room temperature.
Fixative solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 0.2 M Phosphate buffer (pH 7.3) | 100 mM | 25 mL |
| 4% Paraformaldehyde phosphate buffer solution | 1% | 12.5 mL |
| 25% Glutaraldehyde | 0.05% | 100 μL |
| 0.5 M EGTA | 5 mM | 500 μL |
| 1 M MgCl2 | 2 mM | 100 μL |
| 10% NP-40 | 0.1% | 500 μL |
| ddH2O | n/a | 11.3 mL |
| Total | n/a | 50 mL |
Store at 4°C
CRITICAL: Paraformaldehyde solution is a harmful material for humans (Kim et al., 2011). Handling paraformaldehyde solution should be done inside a chemical fume hood and wearing protective gears such as glasses, lab coat, and gloves.
Staining solution
| Reagent | Final concentration | Amount |
|---|---|---|
| 0.2 M Phosphate buffer (pH 7.3) | 0.1 M | 150 mL |
| 1 M MgCl2 | 2 mM | 600 μL |
| 10% Sodium deoxycholate | 0.01% | 300 μL |
| 10% NP-40 | 0.02% | 600 μL |
| 250 mM K-ferricyanide | 5 mM | 6 mL |
| 250 mM K-ferrocyanide | 5 mM | 6 mL |
| 1M Tris-HCl (pH7.3) | 20 mM | 6 mL |
| ddH2O | n/a | 123 mL |
| X-gal (5-Bromo-4-chloro-3-indolyl-β-D-galactopyranoside) stock solution | 1 mg/mL | 7.5 mL (put just before use) |
| Total | n/a | 300 mL |
Store at room temperature and keep the bottle in the dark.
CRITICAL: 250 mM K-ferricyanide and K-ferrocyanide solutions should be prepared at the time of use and keep at room temperature in the dark.
X-gal stock solution
Dissolve 40 mg of X-gal with 1 mL N’N’-dimethylformamide (DMFA) and store at −20°C in the dark until use.
CUBIC-1
| Reagent | Final concentration | Amount |
|---|---|---|
| N,N,N′,N′-Tetrakis(2-hydroxypropyl)ethylenediamine | 25 wt% | 125 g |
| Urea | 25 wt% | 125 g |
| Polyethylene glycol mono-p-iso octylphenyl ether/Triton X-100 | 15 wt% | 75 g |
| ddH2O | n/a | Fill up to 500g |
| Total | n/a | 500 g |
Store at room temperature. Stir at room temperature for a few hours until dissolved and keep on your desk quietly until bubbles disappear.
50% CUBIC-1
| Reagent | Final concentration | Amount |
|---|---|---|
| CUBIC-1 | 50 % | 150 mL |
| ddH2O | 50% | 150 mL |
| Total | n/a | 300 mL |
Anesthetic reagent
| Reagent | Final concentration | Amount |
|---|---|---|
| 1 mg/mL medetomidine | 75 μg/mL | 1.875 mL |
| 5 mg/mL midazolam | 400 μg/mL | 2 mL |
| 5 mg/mL butorphanol | 500 μg/mL | 2.5 mL |
| Saline | n/a | Fill up to 25 mL |
| Total | n/a | 25 mL |
Store at room temperature in the dark. Keep the mixture in the suitable cabinet strictly to avoid touching by irrelevant persons according to the restriction.
Step-by-step method details
Visualization of LacZ activity in murine embryos
Timing: 1–2 h
-
1.Collecting mouse embryos
-
a.Prepare the E15.5 pregnant mice.
-
b.Euthanize a pregnant female by cervical dislocation. Make a cut along the ventral midline of the skin and take both sides of uterine horns into the petri-dish containing 1×PBS.
-
c.Dissect out each implantation site of uterine horn, and carefully remove uterine muscles and the placenta with forceps.
-
d.Transfer embryos surrounded by amniotic membrane from uterus in petri-dish and take embryos out by gently tearing off the yolk sac. Put embryos in each well of a 24-well plate.
-
e.Pour 1 mL of 1×PBS containing Mg2+ into each well and keep on ice until X-gal staining is started.
-
a.
Note: Since removal of Mg2+ has been shown to decelerate the enzymatic activity of LacZ, use 1×PBS containing Mg2+ in this step (Sinnott and Withers, 1974).
Note: The yolk sac can be optionally used for genotyping. If you want to use it for genotyping, do not fix it.
-
2.Fixation and X-gal staining
 Timing: 4–14 h
Timing: 4–14 h
-
a.Put embryos in 1 mL of fixative solution for 2 h at 4°C.
-
b.Wash embryos with 1 mL of 1×PBS containing Mg2+ twice.
-
c.Transfer embryos into 1 mL of staining solution and incubate for 2 h at room temperature with gentle shaking using an in vitro shaker in the dark.
-
d.Wash embryos with 1 mL of 1×PBS twice.
-
e.Incubate embryos in 1 mL of 4% paraformaldehyde/1×PBS for 12 h at 4°C.Note: Ensure that X-gal activity should be displayed as blue color. If the staining is faint, the incubation time can be prolonged up to 12 hours at room temperature with gentle shaking in the dark.
-
a.
-
3.Optically clearing of embryos and imaging
 Timing: 4 days–2 weeks
Timing: 4 days–2 weeks
-
a.Wash embryos with 1 mL of 1×PBS twice.
-
b.Transfer embryos into a 15 mL centrifuge tube, add 10 mL of 50% CUBIC-1, and incubate with gentle shaking at 37°C for 6 h.
-
c.Make sure that the skin of the embryo gets transparent and swollen, then switch to 10 mL of CUBIC-1. Continue incubating with gentle shaking at 37°C.
-
d.Change solution with new 10 mL of CUBIC-1 every second day and continue incubating at 37°C for 14 days until the whole embryo becomes transparent (Figure 1 A).
-
e.Transfer embryos into petri-dish and pour enough CUBIC-1 solution to immerse the whole embryo. Acquire images by stereomicroscopy using transmitted illumination.
 CRITICAL: Fixative solution should be made carefully following this protocol. Excess paraformaldehyde and glutaraldehyde would hamper the original LacZ activity and make embryos yellow, respectively (Figure 1B).
CRITICAL: Fixative solution should be made carefully following this protocol. Excess paraformaldehyde and glutaraldehyde would hamper the original LacZ activity and make embryos yellow, respectively (Figure 1B).
-
a.
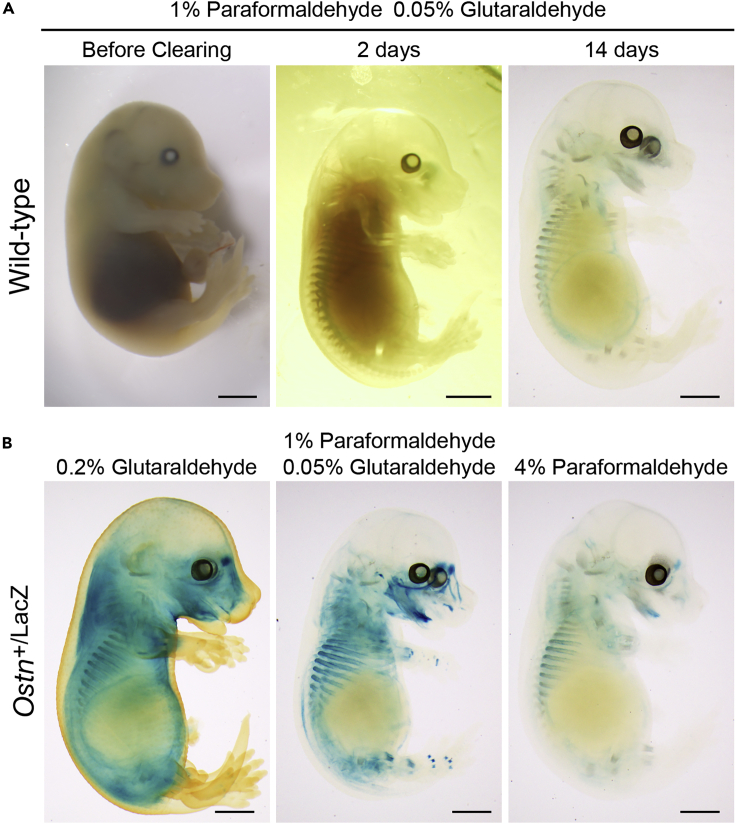
Figure 1.
Appearance of E15.5 embryos after fixation or clearing according to the protocol
(A) E15.5 embryos are fixed with 1% paraformaldehyde and 0.05% glutaraldehyde before cleaning (left), 2 days in CUBIC-1 (middle), and after clearing for 14 days (right). Scale bar, 1 mm.
(B) The final appearance of E15.5 embryos of Ostn+/LacZ fixed with 0.2% glutaraldehyde (left), 1% paraformaldehyde and 0.05% glutaraldehyde (center), and 4% paraformaldehyde (right) for 30 min at 4°C. Note the differences of the LacZ activity in the embryos depending on the fixative conditions. Scale bars, 1 mm. Part of a figure was reused from Watanabe-Takano et al. (2021).
Visualization of LacZ activity in adult mice
Timing: 4–14 h
-
4.Anesthetizing mice and perfusion of whole mouse body at 8 weeks of age
-
a.Prepare all equipment required for dissection, perfusion, and fixation (Figure 2A).
-
b.Prepare an 8-week-old mouse, measure the body weight of the mouse, and anesthetize the mice by injecting 10 μL/g anesthetic reagent intraperitoneally.
-
c.Shave the whole coat of the mouse with an electric clipper (Figure 2B).Note: Fur contamination would directly spoil the appearance of the sample after clearing. Since it is quite hard to remove fur contamination after cleaning, shave the coat of the mice as well as possible in this step.
-
d.Place the mouse in a supine position by putting in a pin through each of the paws and sterilize its abdomen with 70 % EtOH (Figure 2C).
 CRITICAL: The final result depends on this setting, so you should carefully set up the position of the mouse in this step.
CRITICAL: The final result depends on this setting, so you should carefully set up the position of the mouse in this step. -
e.Expose internal organs by cutting the skin and dermis.
-
f.Change gently the position of some organs such as the small intestine, the liver, and so on to fully expose the diaphragm (Figure 2D).
-
g.Make a small incision in the diaphragm with scissors and carefully cut the boundary between the diaphragm and the chest cavity.
-
h.Make an incision in the right atrium and insert a needle into the left ventricle (Figure 2E).Note: Be careful not to break through the left ventricle by your needle, otherwise the whole-body perfusion would not succeed. In addition, please keep the tip of the needle into the left ventricle.
-
i.Perfuse the mouse with more than 25 mL 1×PBS from the left ventricle at a constant flow rate of 5 mL/min. Make sure that the liver of the animal appears pale (Figure 2F).
-
a.
-
5.Fixation and staining of the whole mouse body
 Timing: 2 days
Timing: 2 days
-
a.Fix the mouse by perfusing with 25 mL of fixative solution at the same flow rate as that in perfusion.Note: Ensure the contracture of hind limb muscles and tail in a few minutes after starting perfusion, which is a sign of success.
-
b.Gently remove all the skin from the mouse as well as possible.
-
c.Put the mouse into a plastic bag filled with 150 mL of the fixative solution and sealed the bag by a heat sealer. Make sure that the bubbles are driven out of the bag as possible as you can (Figure 2G).
-
d.Incubate for 45 min with gentle shaking at 4°C (Figure 2H).
-
e.Wash with 150 mL of 1×PBS twice and transfer the mouse body into 150 mL of the staining solution.
-
f.Incubate the sample in the plastic bag filled with staining solution for 12–16 h at room temperature in the dark (Figure 2I).Note: Make sure that the whole-body of the mouse is completely soaked with enough fixative and staining solutions.
-
a.
-
6.Optical clearing of the whole mouse body
 Timing: 2 weeks
Timing: 2 weeks
-
a.Wash with 150 mL of 1×PBS twice and fix again with 150 mL of 4% paraformaldehyde/1×PBS at 4°C for 12 h.
-
b.Wash with 150 mL of 1×PBS twice and transfer the mouse body into 150 mL of 50 % CUBIC-1 at 37°C for 1 day.
-
c.Immerse the mouse in 150 mL of 100% CUBIC-1 and incubate with gentle shaking at 37°C for 2 days.
-
d.Replace the old medium with new 150 mL of CUBIC-1 every two days and keep incubating at 37°C for 14 days with gentle shaking until the mouse becomes transparent.Note: Transparency is confirmed by the decolorization that is caused by heme release from the fixed samples incubated with CUBIC-1 as described previously (Tainaka et al., 2014).
-
e.Sample was taken out from the plastic bag and put in the clear plastic case filled with enough CUBIC-1 to immerse the whole-body of the mouse.
-
f.Take photos of samples illuminated by a light box (Figure 3).
-
a.
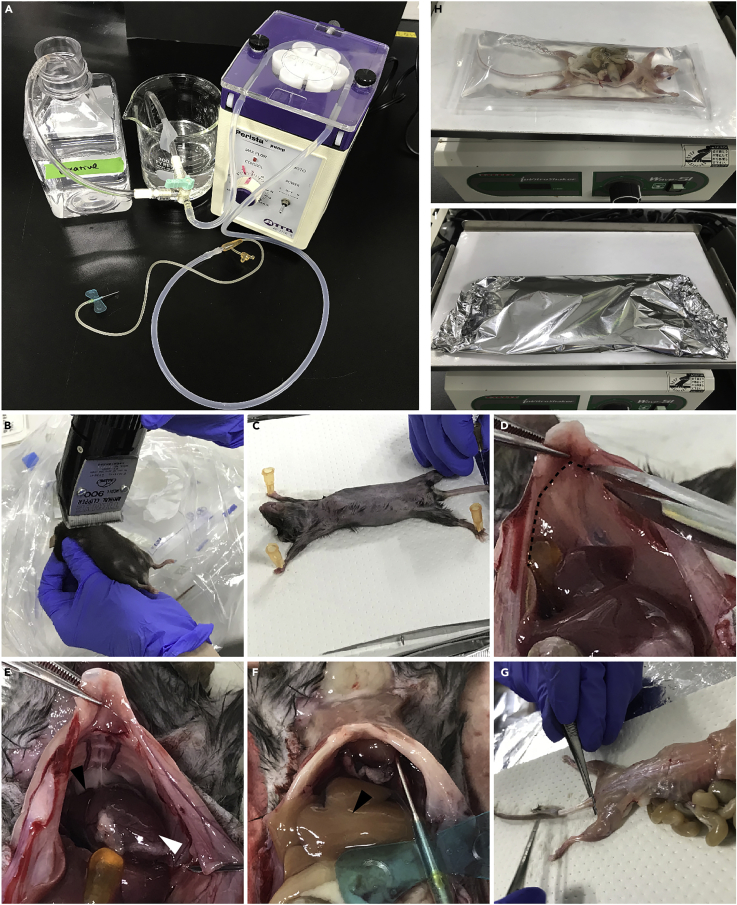
Figure 2.
Detailed method for perfusion, fixation, X-gal staining, and clearing whole-body of the mice at 8 weeks of age
(A) Setting up a peristaltic pump and enough reagents for perfusion and fixation.
(B) Shaving the hair of a mouse with an electric clipper.
(C) Setting the mouse for perfusion by use of the needles.
(D and E) Cutting diaphragm and exposing the chest cavity. Dotted line indicates the border between diaphragm and chest cavity (D). Black and white arrowheads indicate the right atrium and the left ventricle, respectively (E).
(F) Starting perfusion with 1×PBS through the left ventricle. Note the liver appears to be whitish (arrowhead).
(G) Peeling of the skin completely including the tail for X-gal staining.
(H) Fixing the sample with enough amount of the fixative solution with gentle shaking. Note the sample is completely soaked into the fixative solution.
(I) Staining the sample with enough amount of staining solution with gentle shaking wrapped with aluminum foil to keep it in the dark.
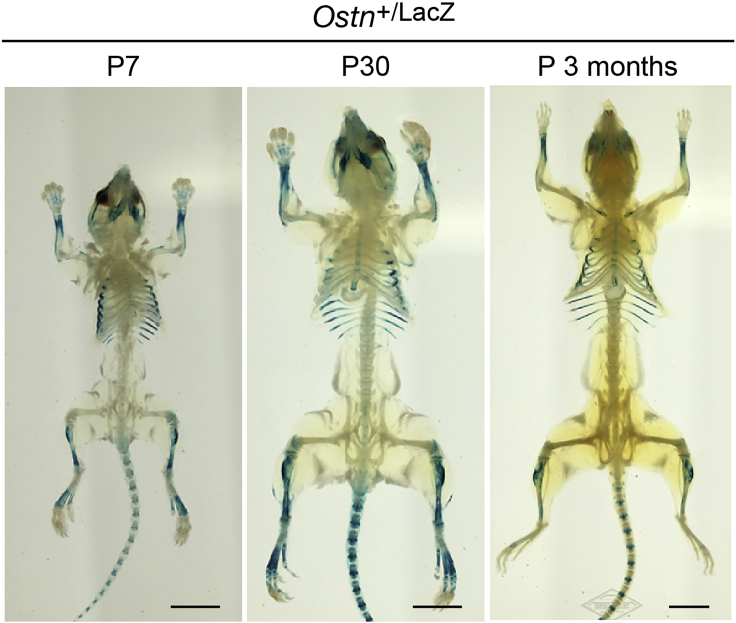
Figure 3.
Examples of the Ostn+/LacZ mouse at P7, P30, and P 3 months of the age following this protocol.
Scale bar, 1 cm. Part of a figure was reused from Watanabe-Takano et al. (2021).
Expected outcomes
This protocol helps in situ expression analysis of the gene of interest during development including embryonic and adult stages of the mouse by combining the traditional X-gal staining with the tissue clearing reagents, CUBIC-1 (Figures 1 and 3) (Tainaka et al., 2014). Furthermore, this protocol enables us to know the endogenous temporal expression of genes of interest using the mice of several developmental stages as well as spatial expression pattern of the gene in whole-body (Figure 3) (Watanabe-Takano et al., 2021). Using this protocol, we investigated the expression of Osteocrin(Ostn)/Muscrin in various developmental stages previously to understand the physiological role of Ostn. In this study, we found that Ostn is highly expressed in the periosteum of the distal bones including tibia, ulna, and radius, but lowly in vertebra, femora, and humeri (Figure 3). Furthermore, we found that Ostn expression peaks at postnatal 30 days and decreased along with the aging. Other expression analyses including qPCR, Laser microdissection, in situ hybridization, supported this result. Since the distal bones might be exposed to the loading caused by weight, gravity, and physical activity, we noticed that the physiological loading might be involved in Ostn expression. Eventually, by use of an unloading model (tail-suspension model), we clarified that the loading is required for maintaining Ostn expression. Accordingly, this protocol enabled us to understand the role for Ostn in loading-induced bone formation. In conclusion, visualizing the whole-body distribution of the gene expression and its changes in the process of development, growth, disease, and aging may help to consider the involvement of genes of your interests and its products in the physiology and pathophysiology.
Limitations
In this protocol, we use the mouse line which was originally generated in our laboratory using the targeting vector provided by KOMP (PG00061_A_2_D09) (Skarnes et al., 2011). Using this line, we found a significant Ostn expression in bones, particularly in the distal bones, but not in other tissues including skeletal muscle (Watanabe-Takano et al., 2021). However, as described in the previous reports, qPCR analysis exactly showed Ostn expression in skeletal muscle, whereas the expression level is lower than that in tibia (Nishizawa et al., 2004; Subbotina et al., 2015). Using this protocol, we detected the expression of another muscle gene in skeletal muscles, suggesting that this protocol can be not only applied for bone, but also for muscle tissues (data not shown). We think that the discrepancies regarding Ostn expression in skeletal muscle could be attributed to the spatial distribution of β-galactosidase in muscle cells, whose size is typically bigger than the cells in other organs, or the expression level is much lower than the limitation of detection. In conclusion, parallel analyses such as immunohistochemistry (IHC), in situ hybridization (ISH), and qPCR using isolated cells and micro-dissected tissues are highly recommended to confirm the distribution of the gene expression. Furthermore, we cannot assure that this protocol can be applied for detecting the cells residing deeply into the tissues. Finally, X-gal staining using the isolated organs should be done in parallel with the whole-mount staining.
Troubleshooting
Problem 1
No or weak signal after X-gal staining.
Potential solution
Make sure that the fixative solution is prepared exactly by the recipe in this protocol. Our previous data show the fixation step is quite critical for detecting LacZ activity (Figure 1B). Having a positive or negative control is necessary for better analysis or interpretation of your results. Alternatively, you may use homozygous for LacZ alleles; however, the phenotypes of homozygous should be taken into account in gene trapping cases.
Problem 2
Uneven staining.
Potential solution
Use enough fixative/staining solutions more than 150 mL per mouse for soaking samples completely. Insufficient fixation/staining often might cause uneven staining. Furthermore, insufficient solution causes stacking of organs, which might inhibit uniform fixative/staining.
Problem 3
Insufficient clearing.
Potential solution
Insufficient perfusion causes bloody organs. Make sure that your perfusion is performed rapidly and well before the formation of blood clots; you will quickly get better results. In that case, prolonged incubation time with QUBIC-1 at 37°C will make the sample more transparent.
Resource availability
Lead contact
Questions and requests for resources and reagents should be directed to Naoki Mochizuki (mochizuki@ncvc.go.jp).
Materials availability
The mouse line B6.129S-OSTNtm1a(KOMP)ncvc, was originally established in Naoki Mochizuki’s laboratory.
Acknowledgments
This work was supported by Grants-in-Aid for Young Scientists (A) (15H05646 to H.W.-T.), for Scientific Research on Innovative Areas (18H04994 to H.W.-T.), and for Scientific Research (C) (18K09050 to H.W.-T.) from Japan Society for the Promotion of Science (JSPS), AMED-CREST (Japan Agency for Medical Research and Development, Core Research for Evolutional Science and Technology) (JP17gm0610010 to N.M.), and grants from the Uehara Memorial Foundation (to H.W.-T), the Mochida Memorial Foundation (to H.W.-T), Meiji Yasuda Life Foundation of and health and welfare (to H.W.-T), and the Okinaka Memorial Institute for Medical Research (to H.W.-T).
Author contributions
H.W.-T. and N.M. designed the experiments and wrote the paper. H.W.-T. performed every analysis. M.F. helped with taking photos and S.F. helped with writing.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Haruko Watanabe-Takano, Email: haruko.watanabe@mpi-bn.mpg.de.
Naoki Mochizuki, Email: mochizuki@ncvc.go.jp.
Data and code availability
This protocol did not generate unique materials or reagents.
References
- Kim K.H., Jahan S.A., Lee J.T. Exposure to formaldehyde and its potential human health hazards. J. Environ. Sci. Health C. Environ. Carcinog. Ecotoxicol. Rev. 2011;29:277–299. doi: 10.1080/10590501.2011.629972. [DOI] [PubMed] [Google Scholar]
- Nishizawa H., Matsuda M., Yamada Y., Kawai K., Suzuki E., Makishima M., Kitamura T., Shimomura I. Musclin, a novel skeletal muscle-derived secretory factor. J. Biol. Chemi. 2004;279:19391–19395. doi: 10.1074/jbc.C400066200. [DOI] [PubMed] [Google Scholar]
- Sinnott L.M., Withers G.S. The β-galactosidase-catalysed hydrolyses of β-d-galactopyranosyl pyridinium salts. Rate-limiting generation of an enzyme-bound galactopyranosyl cation in a process dependent only on aglycone acidity. Biochem. J. 1974;143:751–762. doi: 10.1042/bj1430751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarnes W.C., Rosen B., West A.P., Koutsourakis M., Bushell W., Iyer V., Mujica A.O., Thomas M., Harrow J., Cox T., et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342. doi: 10.1038/nature10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbotina E., Sierra A., Zhu Z., Gao Z., Koganti S.R.K., Reyes S., Stepniak E., Walsh S.A., Acevedo M.R., Perez-Terzic C.M., et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc. Natl. Acad. Sci. U S A. 2015;112:16042–16047. doi: 10.1073/pnas.1514250112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainaka K., Kubota S.I., Suyama T.Q., Susaki E.A., Perrin D., Ukai-Tadenuma M., Ukai H., Ueda H.R. Whole-body imaging with single-cell resolution by tissue decolorization. Cell. 2014;159:911–924. doi: 10.1016/j.cell.2014.10.034. [DOI] [PubMed] [Google Scholar]
- Watanabe-Takano H., Ochi H., Chiba A., Matsuo A., Kanai Y., Fukuhara S., Ito N., Sako K., Miyazaki T., Tainaka K., et al. Mechanical load regulates bone growth via periosteal Osteocrin. Cell Rep. 2021;36:109380. doi: 10.1016/j.celrep.2021.109380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This protocol did not generate unique materials or reagents.