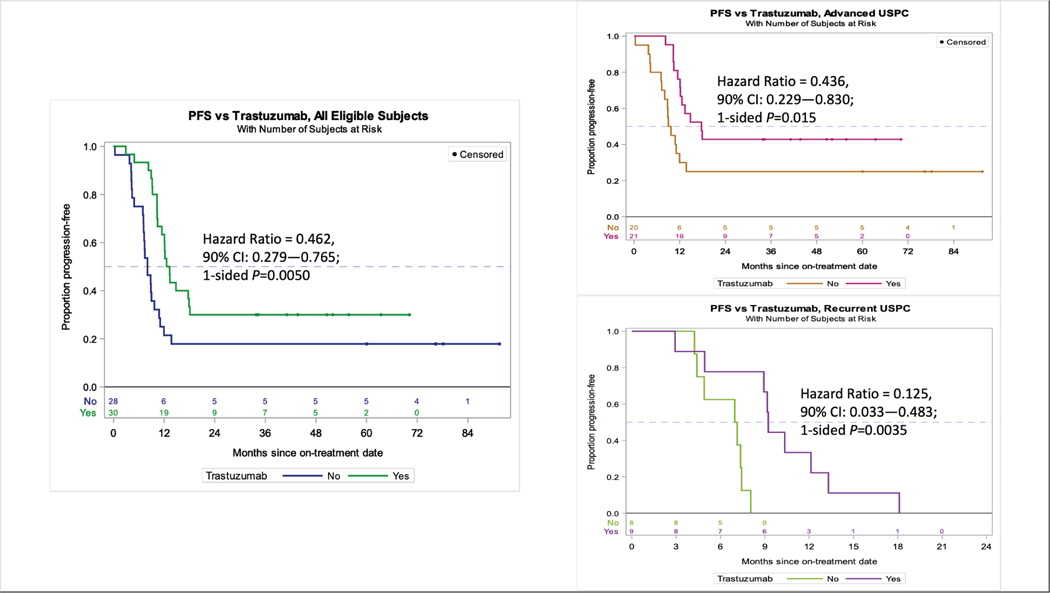
Figure 2.
Updated progression-free survival analyses continue to support the addition of trastuzumab to the treatment of advanced/recurrent uterine serous carcinoma (USC). Left: Median PFS was 8.0 months in patients who received CP and 12.9 months in patients who received CP+T (HR 0.46, 90% CI 0.28–0.76, p=0.005). Benefit from the addition of trastuzumab was greatest in those undergoing primary treatment. Right, top: Median PFS was 9.3 (CP) versus 17.7 (CP+T) months among 41 stage III-IV pts undergoing primary treatment (HR 0.44, 90%CI 0.23–0.83, p=0.015). Right, bottom: Median PFS 7.0 (CP) versus 9.2 (CP+T) months among 17 patients with recurrent disease (HR 0.12, 90%CI 0.03–0.48, p=0.004)