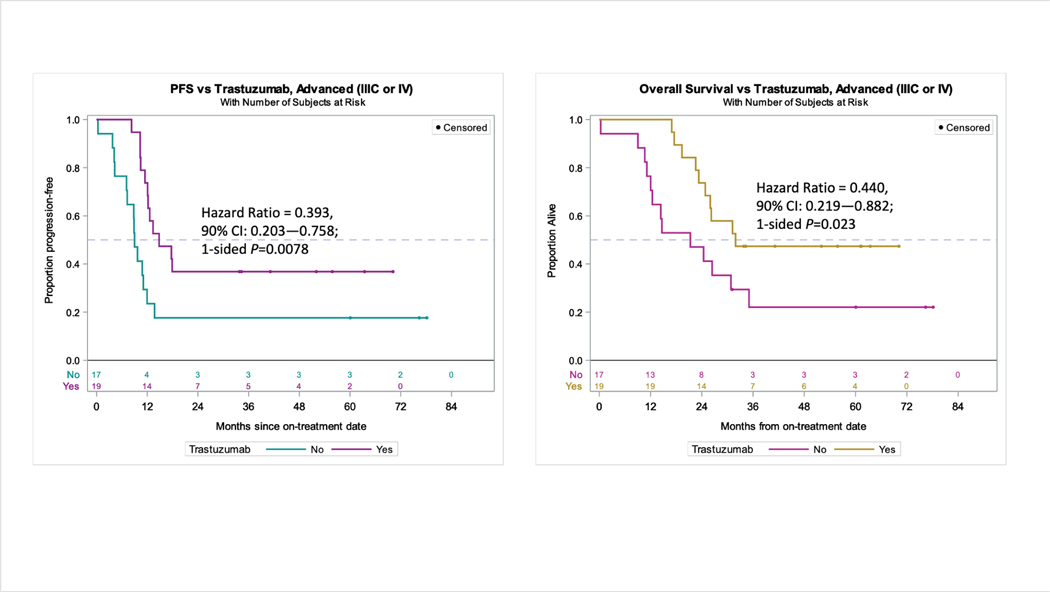
Figure 3.
In a subset analysis of patients restricted to stage IIIC/IV disease, the addition of trastuzumab (n=17) continued to provide both (left) progression-free survival benefit over control (n=19) (9.0 versus 14.8 months, HR 0.393, 90% CI 0.203–0.758, p=0.0078) and (right) overall survival benefit over control (21.1 versus 31.9 months, HR 0.440 90% CI 0.219–0.882 p=0.0230).