Figure 4. VPS26b mediates glutamate receptor trafficking from the recycling endosome.
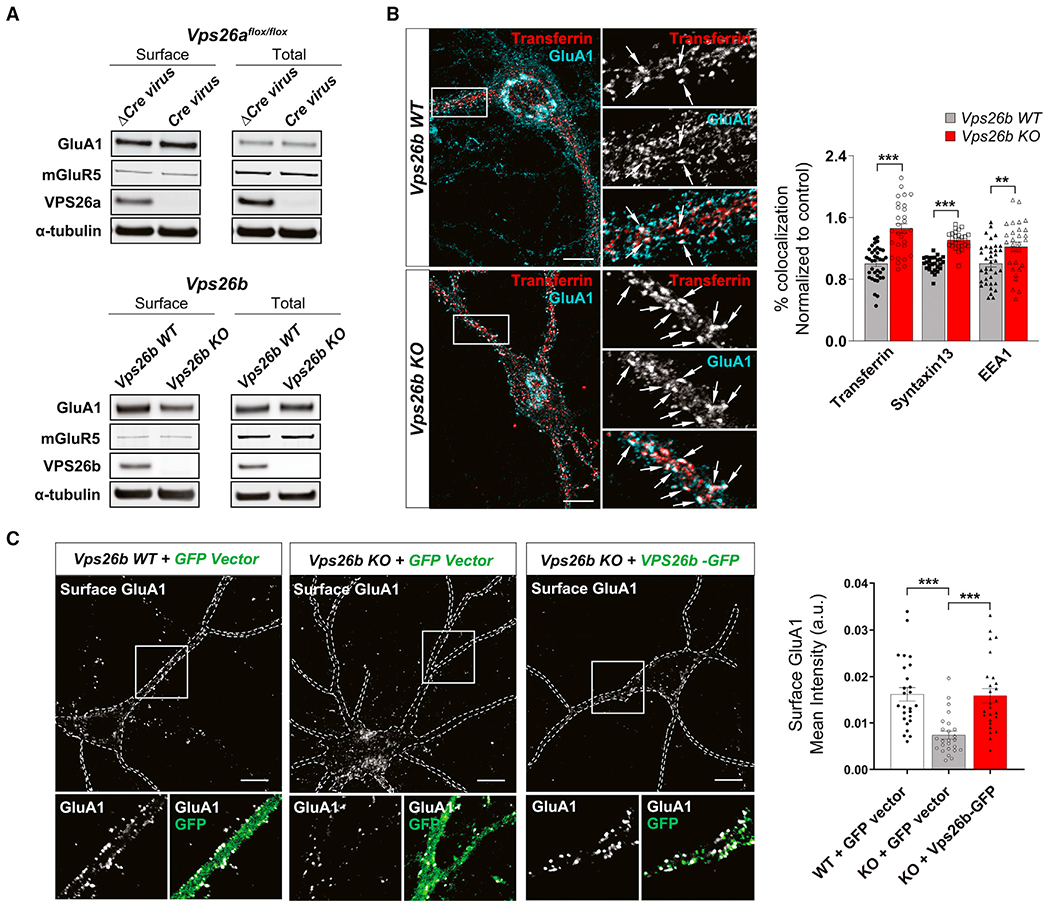
(A) Biotinylated cell-surface proteins were immunoprecipitated from total lysates of cortical neurons depleted for VPS26b (n = 11 biological replicates) or VPS26a (n = 5 biological replicates). Western blot analysis revealed that only VPS26b depletion results in a decrease in GluA1 cell-surface levels.
(B) Representative confocal images of Vps26b KO neurons and Vps26b WT littermates subjected to pulse-chase transferrin uptake and stained for GluA1 and additional endosomal markers. Arrows indicate sites of co-localization. Scale bar, 10 μm (left panel). Quantitative colocalization analysis of GluA1 with early and recycling markers based on Pearson’s correlation coefficients revealed that VPS26b depletion results in an accumulation of GluA1 in recycling endosomes (transferrin: p < 0.0001; Syntaxin13: p < 0.0001) and to a lesser extent in early endosomes (EEA1: p = 0.032) in a non-parametric Kruskal-Wallis with Dunn’s post hoc test (n = 27–37 cells per group/condition, from three separate cultures) (right panel).
(C) Cell-surface GluA1 levels were assessed by confocal microscopy in non-permeabilized cultured neurons incubated with an N-terminal GluA1 antibody in three conditions: Vps26b WT neurons infected with lentivirus expressing GFP alone (“Vps26b WT + GFP vector”), Vps26b KO neurons infected with lentivirus expressing GFP alone (“Vps26b KO + GFP vector”); and Vps26b KO neurons infected with lentivirus expressing VPS26b-GFP (“Vps26b KO + VPS26b-GFP”) (left panel). Scale bar, 10 μm (left panel). Mean fluorescence intensity values revealed that VPS26b repletion fully restored GluA1 surface localization in Vps26b KO neurons, as summarized in the bar graph (p < 0.0001), in a non-parametric Kruskal-Wallis with Dunn’s post hoc test (n = 25–26 neurons/condition, from four independent experiments) (right panel). Data expressed as mean ± SEM., *p < 0.05, **p < 0.01, and ***p < 0.001.
See also Figure S6.
