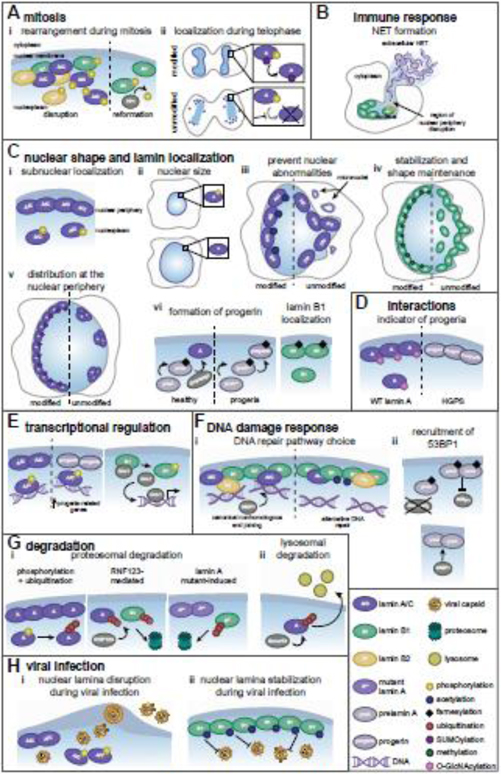
Figure 3. Post-translational modifications regulate lamin functions.
Mature lamin A/C (A/C), lamin A (A), lamin B1 (B1), lamin B2 (B2). (A) (i) During mitosis, lamin phosphorylation promotes nuclear periphery disruption. PP1-mediated B1 dephosphorylation facilitates lamina reformation after mitosis. (ii) At telophase, SUMOylation promotes A dephosphorylation and nuclear relocalization. (B) B1 phosphorylation disrupts the nuclear periphery, releasing chromatin for NET formation by neutrophils. (C) (i) A/C phosphorylation determines sub-nuclear localization. (ii) A phosphorylation maintains nuclear size. (iii) A/C acetylation prevents nuclear deformations. (iv) B1 methylation maintains its localization and nuclear shape. (v) A SUMOylation maintains lamin spacing. (vi) A maturation requires Zmpste24-mediated cleavage of prelamin A and farnesylation removal, which are absent in progeria. Farnesylation promotes proper B1 localization. (D) O-GlcNAcylation decorates wild type A but not progerin. (E) A/C phosphorylation enables its association with enhancers, which is altered by progerin. B1 phosphorylation releases Oct-1. (F) (i) In homeostasis, B1 acetylation increases and decreases associations with lamins and chromatin, respectively. Upon DNA damage, this reduced chromatin association modulates selection of DNA repair pathways. (ii) Farnesylation status of prelamin A impacts 53BP1 recruitment to damaged DNA. (G) (i) A phosphorylation promotes its ubiquitin-mediated degradation, and RNF123-mediated A and B1 ubiquitination induces their degradation. A mutants present in Emery-Dreifuss muscular dystrophy promote B1 ubiquitination-mediated degradation. (ii) Smurf2-mediated ubiquitination is implicated in lysosome-mediated A degradation. (H) (i) A/C phosphorylation during viral infection induces lamina disruption, facilitating viral capsid nuclear egress. (ii) During herpesvirus infection, B1 acetylation inhibits lamina disruption and viral capsid egress.