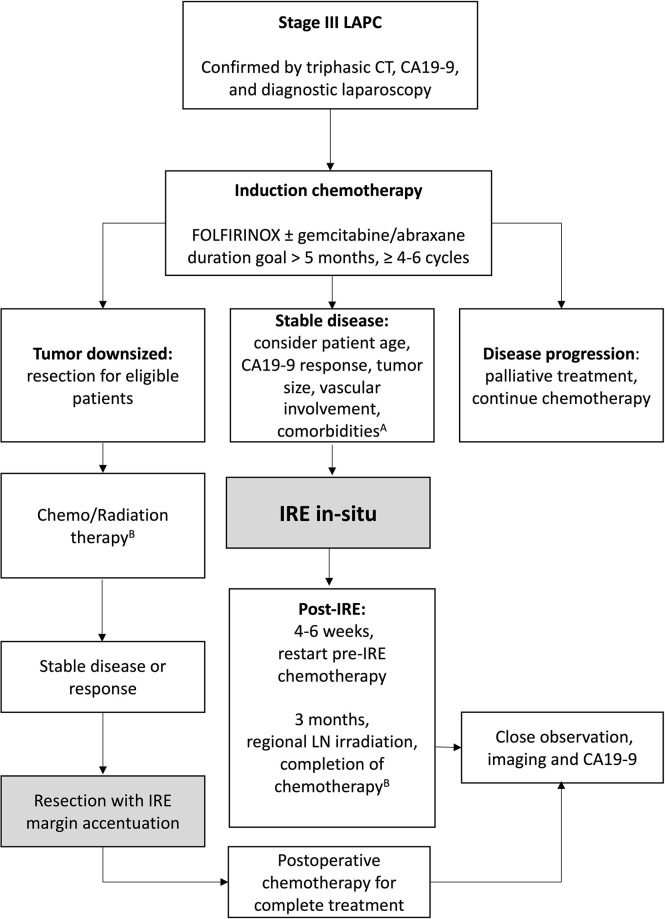
Figure 3.
Patient selection algorithm. A) Optimal patient selection criteria: Age ≤ 61, normalized CA19-9 at IRE, tumor size < 3.6cm, ≤ 180° vascular involvement, less than 2 co-morbidities, and those without diabetes mellitus. B) Can continue FOLFIRINOX (per PRODIGE trial). Chemotherapy goal 12 cycles of FOLFIRINOX, 18 total doses gemcitabine/abraxane.