Abstract
The presence of bronchus-associated lymphoid tissue (BALT) and its size in humans largely depends upon age. It is detected in 35% of children less than 2 years of age, but absent in the healthy adult lung. Environmental gases or allergens may have an effect on the number of BALT. Lungs of rhesus macaque monkeys were screened by histology for the presence, size, and location of BALT after exposure to filtered air for 2, 6, 12, or 36 months or 12 and 36 months to ozone or 2, 12, or 36 months of house dust mite or a combination of ozone and house dust mite for 12 months. In the lungs of monkeys housed in filtered air for 2 months, no BALT was identified. After 6, 12, or 36 months, the number of BALT showed a significantly increased correlation with age in monkeys housed in filtered air. After 2 months of episodic house dust mite (HDM) exposure, no BALT was found. Monkeys exposed to HDM or HDM + ozone did not show a significant increase in BALT compared to monkeys housed in filtered air. However, monkeys exposed to ozone alone did show significant increases in BALT compared to all other groups. In particular, there were frequent accumulations of lymphocytes in the periarterial space of ozone exposed animals. In conclusion, BALT in rhesus monkeys housed under filtered air conditions is age-dependent. BALT significantly increased in monkeys exposed to ozone in comparison with monkeys exposed to HDM.
Keywords: age effects, allergen inhalation, bronchus-associated lymphoid tissue, ozone inhalation, rhesus monkey
1 |. INTRODUCTION
Immune reactions of the lung are of important clinical relevance. Of the roughly 3 million severe lower respiratory tract infections in young children, approximately 265,000 results in acute mortality with 81% of deaths occurring outside a hospital (Nair et al., 2013). Pneumonia is a global burden in children (~120 million episodes) with approximately 18% being caused by Streptococcus pneumonia (Walker et al., 2013). An additional health problem is an increasing incidence of allergic asthma in developed countries (Lambrecht & Hammad, 2015). Infections of the lung have a great influence on the susceptibility to autoimmunity and allergies (Bach, 2009). Therefore, there is a critical need for appropriate animal models to study the pathophysiology and treatment strategies of lung diseases in different age groups. Widely used mouse models have the advantage of an enormous number of genetically modified strains, but several features of lung morphology in mice are different from humans. In mice, there is a monopodial airway branching in contrast to humans that have dichotomous symmetrical branching of airways. Additional differences are present in prenatal and postnatal development (Pabst, 2008). Mice also lack bronchial arteries that are present in humans (Pabst, 2008; Wenzel & Holgate, 2006). These differences warrant the examination of other animal models that duplicate the morphology and development of the human lung (Hein & Griebel, 2003).
Lung morphology and development of rhesus monkeys is similar to human lungs (Plopper & Hyde, 2008). The development of the pulmonary immune system in rhesus monkeys depends on the different cellular components (macrophages, lymphocytes, and neutrophils) present in the developing airways during the postnatal period (Miller, 2004). Several aspects of development and exposure interactions have been studied in rhesus monkeys such as smooth muscle hypertrophy after HDM exposure (Schelegle et al., 2001, 2003; Tran et al., 2004). The advantage of using rhesus monkeys as a model for COPD is summarized in detail (Plopper & Hyde, 2008).
Different routes of vaccination such as intranasal, small aerosol droplets, and large droplets were tested in rhesus monkeys, but histology was not performed to evaluate the potential site of antigen uptake (Bolton et al., 2017). For newborn and adult African green monkeys were infected with influenza virus, the systemic IgG antibody response was similar between age groups (Holbrook et al., 2015). In contrast, the respiratory tract of infant monkeys infected with influenza showed an increased number of Treg cells and volume of bronchus-associated-lymphoid tissue (BALT; Holbrook et al., 2015). Thus, the presence and function of BALT in the African Green monkey model of influenza appears to be age-dependent.
BALT is one of many compartments that make up the immune system of the respiratory tract and has been characterized as a special type of a tertiary lymphoid organ (Pabst, 2007). Human BALT was initially described by Bienenstock and Mc Dermott (2005) and more recently documented by Kawamata et al. (2009). Adhesion molecules of great relevance in lymphocyte migration were expressed in a unique pattern: all high endothelial venules of BALT expressed the addressing for peripheral lymph node and not gut-associated mucosa. Most B cells expressed the α4 and l-selectin in contrast to T lymphocytes (43% α4 integrin and 20% l-selectin; Kawamata et al., 2009). The epithelium of BALT bulges into the bronchial lumen and contains specialized epithelial cells that are similar to the membranous epithelial cells (M cells) of gut-associated lymphoid tissue. M cells function in the uptake of particulate antigens (Wolf & Bye, 1984). In adult humans, BALT is absent (Pabst & Gehrke, 1990), but it is found in approximately 35% of young children (Hiller et al., 2008). The induction of specific protective immunoreactions has been proposed for BALT that is of clinical importance (Pabst & Tschernig, 2010; Wenzel & Holgate, 2006). A different potential role of BALT has been suggested based on studies in rats where BALT expands in pulmonary hypertension and produce autoantibodies (Colvin et al., 2013).
In chronic inflammation and autoimmune diseases, accumulations of lymphoid cells are found in peribronchial, perivascular, and interstitial areas throughout the lung and called induced BALT (iBALT; for review see Randall, 2010; Gilmour et al., 1993). In rhesus monkeys sensitized and challenged with aeroallergen HDM, cellular proliferation studies are consistent with induction of BALT in the wall of bronchi (Miller et al., 2005, 2009). One significant question is whether peribronchial, perivascular, and interstitial iBALT play a similar or differential role in lung pathophysiology. For example, iBALT that forms in the perivascular and/or periarterial space may be of great relevance in lung edema, transplantation, and allergic reactions (Pabst, 2000; Pabst & Tschernig, 2002). In lungs of patients who died in status asthmaticus, an increase of mast cells was also found in the periarterial space (Shiang et al., 2009). In chronic inflammation and autoimmune diseases, accumulations of lung lymphoid cells have often been described as iBALT.
The primary aim of this study was to test whether early-life exposure to ozone or house dust mite (HDM) will induce peribronchial and periarterial BALT in rhesus monkeys. A secondary aim of this study was to evaluate whether the duration of filtered air, ozone, or HDM had an effect on the periarterial accumulation of immune cells in rhesus monkeys.
2 |. MATERIALS AND METHODS
Five micrometers of histologic sections of lung of 49 rhesus monkeys (Macaca mulatta) were obtained from the Respiratory Disease Unit of the California National Primate Research Centre at the University of California, Davis. Each of the rhesus monkeys was enrolled in one of several previously published studies (Chou et al., 2005; Joad et al., 2008; Kawamata et al., 2009; Miller et al., 2005; Moore et al., 2014; Murphy et al., 2012; Schelegle et al., 2003). These studies examined the effect of exposure to filtered air, ozone, house dust mite (HDM), or the combination of ozone plus HDMA. The studies varied in whether the rhesus monkeys were sensitized to house dust mite, the specific sensitizing HDM used (Der f or Der p), the age at which sensitization and exposure occurred, and the age at necropsy (Table 1). The HDM sensitization was confirmed by skin testing (for details see Schelegle et al., 2003). The protocol of the exposure is shown in Figure 1. All protocols were approved by the University of California, Davis Institutional Animal Care and Use Committee in compliance with the Animal Welfare Act and Public Health Service Policy on Human Care and Use of Laboratory Animals. Monkeys selected for these studies were the California National Primate Research Center colony born rhesus macaques. Care and housing of animals before, during, and after treatment was performed by the California National Primate Research Center, which is accredited by the Association for Accreditation and Assessment of Laboratory Animal Care (AAALAC).
TABLE 1.
BALT per section, age, number of animals and sections evaluaten.
| Exposurec,d | Age (months) | Number of animals | Number of BALT/section (Mean ± SD) | Number of sections evaluated |
|---|---|---|---|---|
| Filtered air | 2 | 3 | 0 | 32 |
| 6 | 6 | 0.42 ± 0.33 | 60 | |
| 12 | 4 | 0.71 ± 0.48a,c,d | 78 | |
| 36 | 6 | 0.82 ± 0.48b,e | 58 | |
| HDM | 2 | 3 | –, – | 25 |
| 12 | 6 | 0.38 ± 0.35c | 63 | |
| 36 | 6 | 1.0 ± 0.78e | 55 | |
| Ozone | 12 | 3 | 3.0 ± 1.0c | 29 |
| 36 | 3 | 10.0 ± 5.3e | 24 | |
| HDM + Ozone | 12 | 6 | 1.44 ± 0.73c | 52 |
Correlation of BALT with ages 2–12 months, P = 0.01.
Correlation of BALT with ages 2–36 months, P = 0.05.
Krushal-Wallis ANOVA for BALT at 12 months, all exposures, P = 0.005.
Dwass-Steel-Chritchlow-Fligner test for BALT at 12 months, for Filtered Air vs HDM, P = 0.001, for Filtered Air vs Ozone, P = 0.001.
Krushal-Wallis ANOVA for BALT at 36 months, all exposures, P = 0.032.
FIGURE 1.

The house-dust-mite and ozone exposure of the monkeys (for details see Joad et al. (2008), Kajekar et al. (2007), Moore et al. (2014), Murphy et al. (2012), Schelegle et al. (2003))
The lungs were fixed by 30 cm H2O inflation with glutaraldehyde/paraformaldehyde (1%/1%) and sliced perpendicular to the long axis of the intrapulmonary conducting airways. (Figure 2) The blocks of one lobe were numbered L1 – L9. As in previous studies with these rhesus monkeys, one lobe is representative of the whole lung. The 5 μm sections were stained with hematoxylin and eosin. The microscopic evaluation was performed at 10 times magnification. For some details, 25 times magnification was used. The total area of the sections was quantified by a caliper. The mean evaluated area per section was 1.2 cm2. The periarterial space was also screened for leukocyte infiltration.
FIGURE 2.
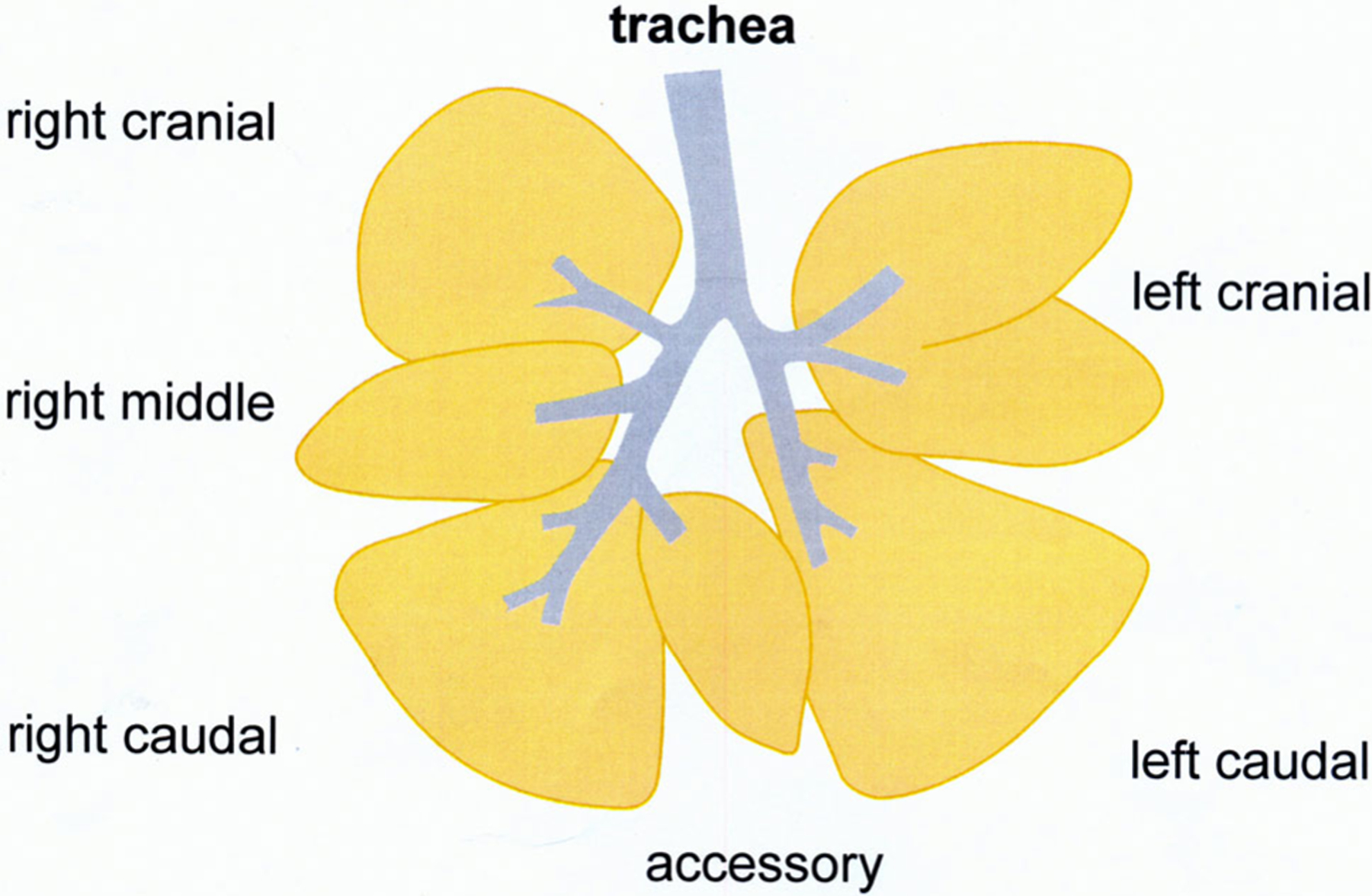
Schematic drawing of the lung of rhesus monkeys
The total number of BALT in the different parts of the lung was counted (L1–L9). L1 was the sample nearest to the lobe bifurcation and L9 the most distal airway sample. The mean ± standard error of the mean (SEM) were evaluated. Statistical analysis of BALT per section was evaluated using Systat 13.1 (Systat Software Inc., San Jose, California, www.systatsoftware.com) by age and relative to the filtered air group using Pearson Correlation Coefficient. Group and age comparisons were done using Kruskal–Wallis one-way analysis of variance and Dwass–Steel–Chritchlow–Fligner test for all pairwise comparisons.
3 |. RESULTS
Lungs of monkeys from all groups were optimally fixed, enabling the identification of BALT by low power magnification. Typical BALT was identified in proximal parts of the bronchial tract (Figure 3) as well as the more peripheral parts (Figure 4). The numbers of sections evaluated in each group are shown in Table 1. In lungs of monkeys housed in filtered air for 2 months, no BALT was identified. The data between animals within one treatment group varied. However, several differences reached the level of statistical significance as indicated in the legend of the table. After 6, 12, or 36 months, the number of BALT showed a significantly increased correlation with age (Table 1). After 2 months of episodic exposure to HDM, no BALT was found. Monkeys exposed to HDM or HDM + ozone did not show significant increases in BALT compared to filtered air monkeys. However, monkeys exposed to ozone alone did show significant increases in BALT compared to other animal groups. Further, after ozone exposure for 12 months, periarterial leukocyte infiltrations were observed (data not shown).
FIGURE 3.

BALT in the lung of a rhesus monkey proximal part of the lung after breathing filtered air for 12 months. Insert higher magnification
FIGURE 4.

BALT in the peripheral parts of the lung of a rhesus monkey breathing house-dust-mite and ozone (12 months). The insert documents the BALT at on higher magnification
4 |. DISCUSSION
The factors responsible for the induction of BALT are unknown. One suspected microbial agent is mycoplasma because it is well documented to be a causative agent in pigs; Sarradell and colleagues described the lymphocyte subsets and macrophages in BALT in the lung of naturally infected pigs with Mycoplasma pneumoniae (Sarradell et al., 2003). In calves experimentally infected with Mycoplasma pneumoniae, BALT was hyperplastic with cells expressing TNFα, IL-4, and IFNg (Rodríguez et al., 2015). The lungs of mice with lymphoid infiltrates also have increased numbers of cytokine-producing T-cell (Shilling et al., 2013). It is not known whether the presence of BALT is positive or negative with regard to human lung disease. In chronic obstructive pulmonary disease (COPD), BALT is found with increasing incidence with the stage of the disease (Holbrook et al., 2015).
In contrast with humans, we observed that the number of BALT in rhesus monkeys housed under filtered air conditions significantly increased with age. It cannot be excluded that lipopolysaccharide (LPS) was present in filtered air housing for rhesus monkeys. LPS administration as a model for acute lung injury by the intraperitoneal route in adult rats showed that 18 hr following treatment, BALT hyperplasia was found in 10 out of 13 animals (Bánfi et al., 2009). A different exposure protocol to HDM and/or ozone might be tested. There are no data known to us on the effects on the bronchus epithelium which might explain the difference between monkeys exposed to ozone alone and ozone + HDM. Ozone and HDM exposure did not increase the number of BALT. In future experiments, the TLR2/6 agonist MALP-2 might be an option to induce BALT in rhesus monkeys and then test these structures as entry sites for antigens for vaccinations as suggested previously (Pabst & Tschernig, 2010).
Holbrook et al. (2015) studied the influenza-specific antibody response in infant and adult African green monkeys. The infection in infants resulted in more pathology and higher viral load compared to adults. The lower response in adults was associated with an increased prevalence of T-Reg and lowered levels of BALT (Holbrook et al., 2015). Thus, it is not clear whether the presence of BALT was advantageous or not. BALT had been induced and therefore called iBALT (for review see Randall (2010)). Some of these accumulations for lymphoid cells, however, were not localized in the wall of a bronchus and therefore the term bronchus-associated is misleading. The accumulation of lymphoid cells in different organs is called “tertiary lymphoid organs” (Pabst, 2007). We previously proposed to differentiate BALT either as a normal, physiological tertiary lymphoid organ or a pathological form (Pabst & Tschernig, 2010). More recently interesting new data here been published for iBALT in the mouse. There is a synergism between iBALT and local draining lymph nodes during an antiviral CD4 T-cell response. This is a further observation for the presence of lymphatics running from iBALT to draining nodes (Richert et al., 2013). Thus, iBALT seems to play a much more relevant role in lung diseases than previously thought.
Ozone is known to interfere with the development of distal airways in infant rhesus monkeys (Fanucchi et al., 2006) and the frequency of immune cells in the lung (Miller et al., 2009). Chronic exposure to ozone had negative effects on lung function in young adults (Tager et al., 2005). The significant increase of BALT in monkeys breathing ozone alone in the present study can be interpreted as a positive reaction of the organism to increase antigen uptake as discussed previously (Pabst & Tschernig, 2002).
The periarterial space of the lungs is a compartment for lymphoid cells in the lung (Pabst, 2000). Normally this space is more or less free of immune cells. However, in allergic and inflammatory reactions, immune cells rapidly accumulate here (for review see Pabst and Tschernig (2002)). In the lung of patients who died of fatal asthma, the periarterial space showed significantly more mast cells, eosinophils, and neutrophils, but no significant differences were observed in B- and T-cell subsets (Shiang et al., 2009). In the present study, phenotyping of immune cells in the perivascular space of rhesus monkeys could not be performed on H & E stained sections. Future studies might include immunohistochemistry similar to that described by Miller et al. (2005), which documented an increase of CD4 T cells, CD25+ cells, dendritic cells, and eosinophils after HDM challenge in the bronchial wall of rhesus monkeys. Moreover, there were distinct differences with respect to a greater frequency of immune cells in more peripheral parts of the bronchial tract (Miller et al., 2005).
In conclusion, in this study, BALT development was age-dependent on rhesus monkeys in contrast to humans. Thus, the rhesus monkey may not be an optimal animal model for the study of human BALT in this respect.
ACKNOWLEDGMENTS
The secretarial help of Sabine Buhmann and Silke Wallbaum as well as the preparation of the figures by Marita Peter are gratefully acknowledged. R. P. had been supported by the German Research Foundation (DFG; SFB 587). Animal work was supported by NIH P01ES011617 and P51OD011107.
Funding information
National Institutes of Health, Grant/Award Numbers: P51OD011107, P01ES011617; German Research Foundation, Grant/Award Number: SFB 587
REFERENCES
- Bach JF (2009). The effect of infections on susceptibility to autoimmunity and allergic diseases. The New England Journal of Medicine, 347, 911–920. [DOI] [PubMed] [Google Scholar]
- Bánfi A, Tiszlavicz L, Székely E, Peták F, Tóth-Szüki V, Baráti L, … Novák Z (2009). Development of bronchus-associated lymphoid tissue hyperplasia following lipopolysaccharide-induced lung inflammation in rats. Experimental Lung Research, 35, 186–197. [DOI] [PubMed] [Google Scholar]
- Bienenstock J, & Mc Dermott MR (2005). Bronchus- and nasal-associated lymphoid tissues. Immunological Reviews, 206, 22–31. [DOI] [PubMed] [Google Scholar]
- Bolton DL, Song K, Thomaras GD, Rao S, & Roederer M (2017). Unique cellular and humoral immunogenicity profiles generated by aerosol, intranasal, or parenteral vaccination in rhesus macaques. Vaccine, 35, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DL, Daugherty BL, McKenna EK, Hsu WM, Tyler NK, Plopper CG, … Miller LA (2005). Chronic aeroallergen during infancy enhances eotaxin-3 expression in airway epithelium and nerves. American Journal of Respiratory Cell and Molecular Biology, 33, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, & Yeager ME (2013). Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. American Journal of Respiratory and Critical Care Medicine, 188, 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, van Winkle LS, Gershwin LJ, & Schelegle ES (2006). Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. American Journal of Physiology. Lung Cellular and Molecular Physiology, 291, L644–L650. [DOI] [PubMed] [Google Scholar]
- Gilmour MI, Park P, Doerfler D, & Selgrade MK (1993). Factors that influence the suppression of pulmonary antibacterial defenses in mice exposed to ozone. Experimental Lung Research, 19, 299–314. [DOI] [PubMed] [Google Scholar]
- Hein WR, & Griebel PJ (2003). A road less travelled: large animal models for immunological research. Nature Reviews. Immunology, 3, 79–84. [DOI] [PubMed] [Google Scholar]
- Hiller AS, Tschernig T, Klemann WJ, & Pabst R (2008). Bronchus-associated lymphoid tissue (BALT) and larynx-associated lymphoid tissue (LALT) are found in different frequencies in children, adolescents and adults. Scandinavian Journal of Immunology, 47, 159–162. [DOI] [PubMed] [Google Scholar]
- Holbrook BC, Hayward SL, Blevins LK, Kock N, Aycock T, Parks GD, & Alexander-Miller MA (2015). Nonhuman primate infants have an impaired respiratory but not systemic IgG antibody response following influenza virus infection. Virology, 476, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joad JP, Kot KS, Bric JM, Schelegle ES, Gershwin LJ, & Plopper CG (2008). The effects of inhaled corticosteroids on intrinsic responsiveness and histology of airways from infant monkeys exposed to house dust mite allergen and ozone. Toxicology and Applied Pharmacology, 226, 153–160. [DOI] [PubMed] [Google Scholar]
- Kawamata N, Xu B, Nishijima H, Aoyama K, Kusomoto M, Takeychi T, … Matsuyama T (2009). Expression of endothelial and lymphocyte adhesion molecules in bronchus-associated lymphoid tissue (BALT) in adult human lung. Respiratory Research, 10, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajekar R (2007). Environmental factors and devolpmental outcomes in the lung. Pharmacol Therap, 118, 128–145. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, & Hammad H (2015). The immunology of asthma. Nature Immunology, 16, 45–56. [DOI] [PubMed] [Google Scholar]
- Miller LA (2004). Development of the pulmonary immune system. In Harding R, Pinkerton KE, & Plopper CO (Eds.), The lung: Development, aging and environment (pp. 169–185). Amsterdam: Elsevier. [Google Scholar]
- Miller LA, Gerreits JE, Tyler NK, Abel K, Schelegle E, Plopper CG, & Hyde DM (2009). Ozone and allergen exposure during post natal development alters the frequency and airway distribution of DC25+ cells in infant rhesus monkeys. Toxicology and Applied Pharmacology, 236, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Hurst SD, Coffman RL, Tyler NK, Stovall MY, Chou DL, … Hyde DM (2005). Airway generation-specific differences in the spatial distribution of immune cells and cytokines in allergen-challenged rhesus monkeys. Clinical and Experimental Allergy, 35, 894–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BD, Hyde DM, Miller LA, Wong EM, & Schelegle ES (2014). Persistence of serotonergic enhancement of airway response in a model of childhood asthma. American Journal of Respiratory Cell and Molecular Biology, 51, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SR, Schelegle ES, Edwards PC, Edwards PC, Miller LA, Hyde DM, & van Winkel LS (2012). Postnatal exposure history and airways: Oxidant stress responses in airway explants. American Journal of Respiratory Cell and Molecular Biology, 47, 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Simões EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JS, … Severe Acute Lower Respiratory Infections Working Group. (2013). Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet, 381, 1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R (2000). Mucosa associated lymphoid tissue of the lung: localization, numbers and dynamics of lymphoid cells in the five different compartments. In Busse WW & Holgate ST (Eds.), Asthma and Rhinitis (pp. 543–546). Oxford: Blackwell. [Google Scholar]
- Pabst R (2007). Plasticity and heterogeneity of lymphoid organs. What are the criteria to call a lymphoid organ primary, secondary or tertiary? Immunology Letters, 112, 1–8. [DOI] [PubMed] [Google Scholar]
- Pabst R (2008). Are animal models of asthma useful? In Kay AB, Kaplan AP, Bousquet J, & Holt PG (Eds.), Allergy and Allergic Diseases (2nd ed., pp. 1214–1222). Oxford: Blackwell. [Google Scholar]
- Pabst R, & Gehrke I (1990). Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals inclucing humans? American Journal of Respiratory Cell and Molecular Biology, 3, 131–135. [DOI] [PubMed] [Google Scholar]
- Pabst R, & Tschernig T (2002). Perivascular capillaries in the lung: An important but neglected vascular bed in immune reactions? The Journal of Allergy and Clinical Immunology, 110, 209–214. [DOI] [PubMed] [Google Scholar]
- Pabst R, & Tschernig T (2010). Bronchus-associated lymphoid tissue: an entry site for antigens for successful mucosal vaccinations? American Journal of Respiratory Cell and Molecular Biology, 43, 137–141. [DOI] [PubMed] [Google Scholar]
- Plopper CG, & Hyde DM (2008). The non-human primate as a model for studying COPD and asthma. Pulmonary Pharmacology & Therapeutics, 21, 755–766. [DOI] [PubMed] [Google Scholar]
- Randall TD (2010). Bronchus-associated lymphoid tissue (BALT) structure and function. Advances in Immunology, 107, 187–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert LE, Harmsen AL, Rynda-Apple A, Wiley JA, Servid AE, Douglas T, & Harmsen AG (2013). Inducible bronchus-associated lymphoid tissue (iBALT) synergizes with local lymph nodes during antiviral CD4+ T cell responses. Lymphatic Research and Biology, 11, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez F, Castro P, Poveda JB, Afonso AM, & Fernández A (2015). Immunohistochemical labelling of cytokines in calves infected experimentally with Mycoplasma bovis. Journal of Comparative Pathology, 152, 243–247. [DOI] [PubMed] [Google Scholar]
- Sarradell J, Andrada M, Ramírez AS, Fernández A, Gómez-Villamandos JC, Jover A, … Rodrigez F (2003). A morphologic and immunohistochemical study of the bronchus-associated lymphoid tissue of pigs naturally infected with Mycoplasma hyopneumoniae. Veterinary Pathology, 40, 395–404. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Gershwin LJ, Miller LA, Fanucchi MV, van Winkle LS, Gerriets JP, … Plopper CG (2001). Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae). The American Journal of Pathology, 158, 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, van Winkle LS, Gerriets JE, … Plopper CG (2003). Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in rhesus monkeys. Toxicology and Applied Pharmacology, 191, 74–85. [DOI] [PubMed] [Google Scholar]
- Shiang C, Mauad T, Senhorini A, de Araújo BB, Ferreira DS, da Silva LF, … Pabst R (2009). Pulmonary periarterial inflammation in fatal asthma. Clinical and Experimental Allergy, 39, 1499–1507. [DOI] [PubMed] [Google Scholar]
- Shilling RA, Williams JW, Perera J, Berry E, Wu Q, Cummings OW, … Huang H (2013). Autoreactive T and B cells induce the development of bronchus-associated lymphoid tissue in the lung. American Journal of Respiratory Cell and Molecular Biology, 48, 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager IB, Balmes J, Lurmann F, Ngo L, Alcorn S, & Künzli N (2005). Chronic exposure to ambient ozone and lung function in young adults. Epidemiology, 16, 751–759. [DOI] [PubMed] [Google Scholar]
- Tran MU, Weir MV, Fanucchi AE, Murphy AE, van Winkle LS, Evans MJ, … Plopper CG (2004). Smooth muscle hypertrophy in distal airways of sensitized infant rhesus monkeys exposed in house dust mite allergen. Clinical & Experimental Allergy, 34, 1627–1633. [DOI] [PubMed] [Google Scholar]
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, … Black RE (2013). Global burden of childhood pneumonia and diarrhoea. Lancet, 381, 1405–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel S, & Holgate ST (2006). The mouse trap. It still fields few answers in asthma. American Journal of Respiratory and Critical Care Medicine, 174, 1173–1175. [DOI] [PubMed] [Google Scholar]
- Wolf JL, & Bye WA (1984). The membranous epithelial (M) cell and the mucosal immune system. Annual Review of Medicine, 35, 95–112. [DOI] [PubMed] [Google Scholar]


