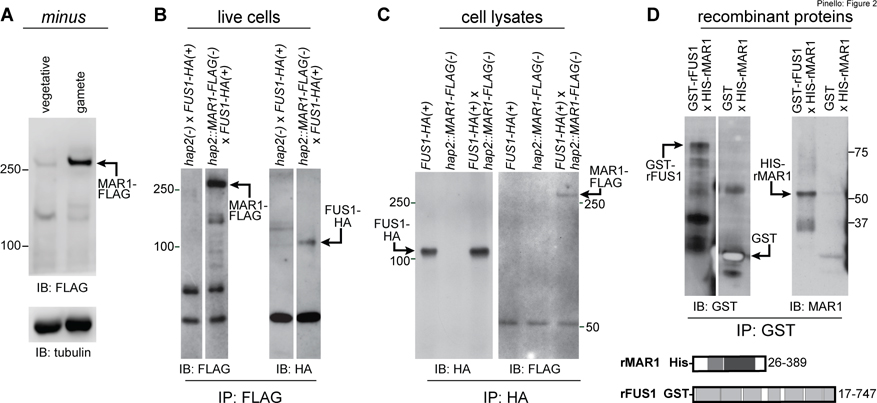
Figure 2. MAR1 on minus gametes directly interacts with FUS1 on plus gametes.
(A) MAR1-FLAG is enriched in minus gametes compared to minus vegetative cells. Immunoblot (IB) with anti-FLAG antibodies showing equal cell equivalents of hap2::MAR1-FLAG; HAP2-HA(−) vegetative cells and gametes (upper panel). Lower panel shows a tubulin loading control. (B) MAR1-FLAG interacts with FUS1-HA during mating structure adhesion. FUS1-HA(+) gametes were mixed for 30 min with hap2::MAR1-FLAG(−) or hap2(−) gametes, and lysate supernatants from the mixed gametes were subjected to immunoprecipitation (IP) with anti-FLAG antibodies followed by immunoblotting with anti-FLAG (left) or anti-HA antibodies (right). (C) FUS1 and MAR1 interact with each other when separately prepared cell lysates are mixed. Separately lysed mixtures of FUS1-HA(+) with hap2(−) gametes and hap2::MAR1-FLAG(−) with fus1(+) gametes were either kept separate (controls; left and middle lanes) or mixed together (right lane) and subjected to immunoprecipitation with anti-HA antibodies followed by immunoblotting with anti-HA (left) or anti-FLAG (right) antibodies (see also Fig. S2). (D) The recombinant GST-tagged ectodomain of FUS1 (GST-rFUS1) interacts with the recombinant His-tagged ectodomain of MAR1 (His-rMAR1). GST-rFUS1 and GST protein in bacterial lysates were bound to glutathione beads followed by incubation of those beads with lysates from bacteria expressing His-rMAR1. Proteins eluted with reduced glutathione were immunoblotted with anti-GST (upper left) or anti-MAR1 antibodies (upper right). Illustrations depicting the ectodomain residues in His-rMAR1 and GST-rFUS1 recombinant proteins (lower panel). White spaces indicate superfluous lanes digitally eliminated in blots in B and D. Protein interaction experiments were carried out at least 3 times.