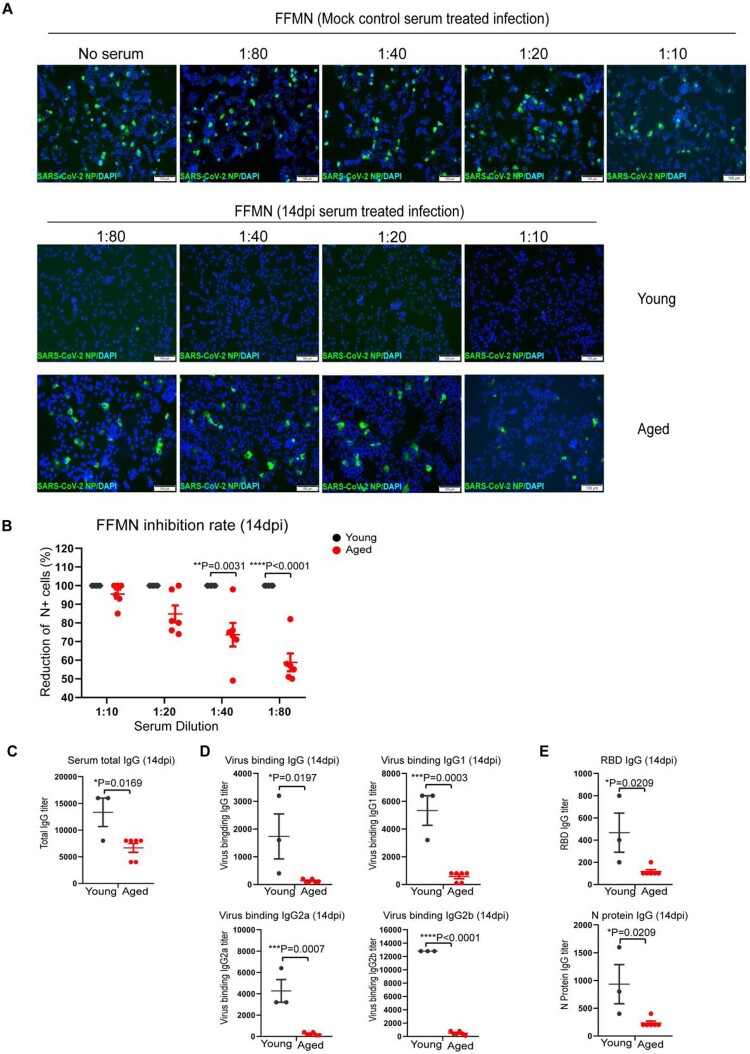
Figure 3.
Serum antibody titres in SARS-CoV-2 B.1.1.7 infected aged and young mice determined by FFMN assay and ELISA. Fourteen days after intranasal inoculation of the B1.1.7 virus, the serum samples were taken for antibody determination, viral-neutralizing antibody determination on Vero E6 cells using fluorescence foci microneutralization assay (FFMN). Serum IgG and viral binding IgG were determined by ELISA with viral antigen-coated plates. (A) Representative images of immunofluorescence-stained SARS-CoV-2 NP in FFMN assay. SARS-CoV-2 B.1.1.7 virus (M.O.I. = 0.1) was allowed to react with the 2-fold serial diluted sera for one hour at 37°C before being added to Vero E6 cells. The cells were fixed and stained for SARS-CoV-2 NP after 6 h of incubation. Mock control mouse serum was tested parallel and shown in the top panel. The image in the middle panel showed no virus NP-positive cells in young mouse serum-treated infections, while the abundant NP-positive cells could be seen in aged mouse serum-treated infection (lower panel). (B) Percentage of reduction of N-positive cell after different mouse sera-treated infection versus mock controls in FFMN assay. (C–E) Mouse serum total IgG antibody (C), viral binding IgG and IgG subtypes (IgG1, IgG2a and IgG2b) (D) and IgG against RBD and N protein (E) in mouse serum determined by ELISA. Data represent mean ± SEM. n = 3–6 for each group. *P < 0.05, ***P < 0.001, ****P < 0.0001 by Student’s t-test.