Fig. 1. Study design and measured levels of PFOA and GenX in the serum and urine of developmentally exposed offspring at postnatal day (PND) 5.5.
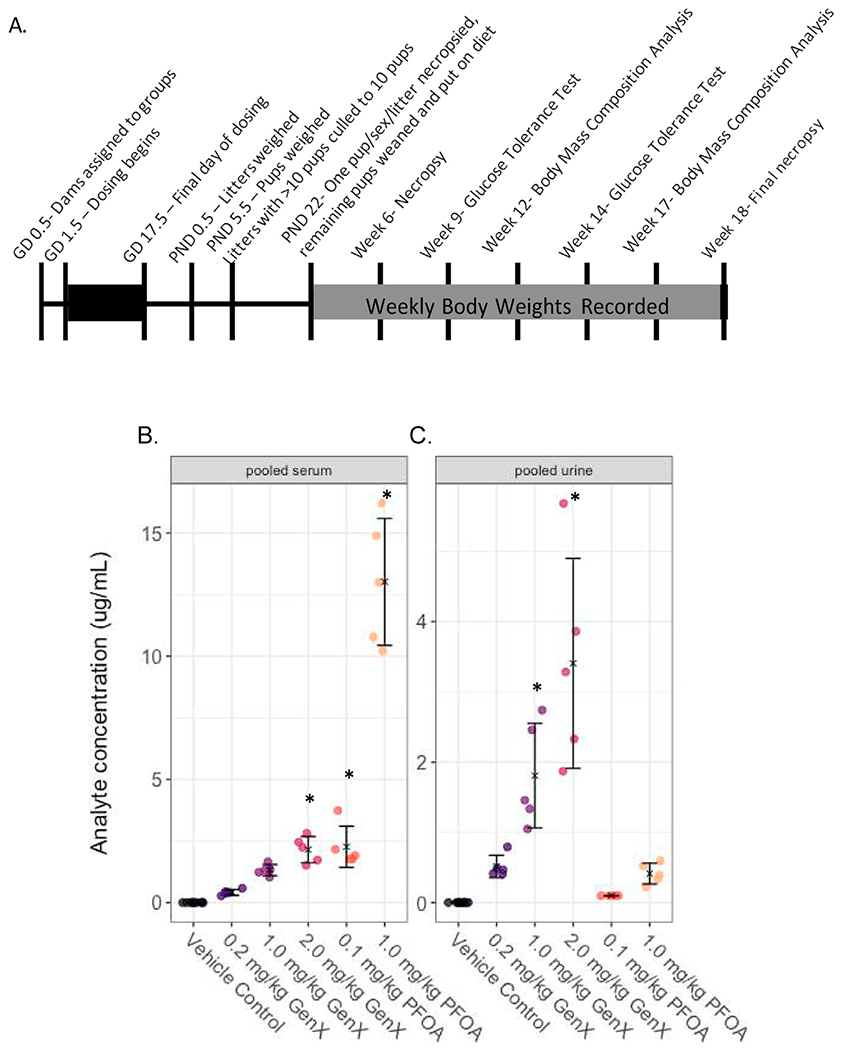
(A) The study design encompassed about 22 weeks from start to finish. (B) PFOA or GenX concentrations in samples obtained from pooling serum from littermates (μg analyte/mL serum) and (C) PFOA or GenX concentration in samples obtained from pooling urine from littermates (μg analyte/mL urine) were determined by high performance liquid chromatography tandem mass spectrometry. Treatment group mean values are denoted with an “X” flanked above and below by error bars showing standard deviation and individual data points are shown as circles (N = 5 litters per group). Note: Vehicle control (VC) samples were quantified for PFOA and GenX. Vehicle control samples were run against a low concentration calibration curve with a limit of detection of 5 ng/mL. GenX and PFOA experimental samples were run against higher calibrations curve with a LOD of 100 ng/mL. All vehicle control samples were below the LOD of 5 ng/mL for both PFOA and GenX and all 0.1 mg/kg PFOA samples were below the LOD of 100 ng/mL for PFOA. Sufficient serum and urine sample quantities were achieved by pooling across pups within the same litter. Statistical comparisons across all treatment groups for serum and urine samples are shown in Supplemental Table 3.
