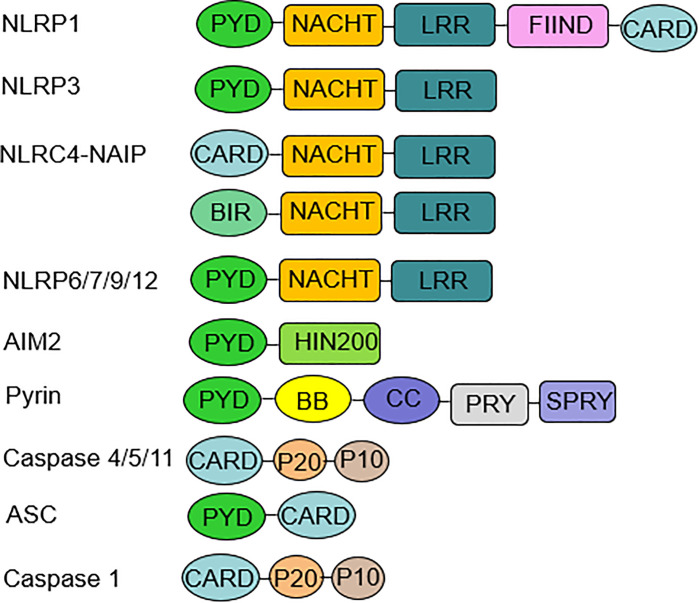
Figure 1.
Inflammasome components and domain structure. Inflammasome complexes are formed by the oligomerization of several protein domains. A typical inflammasome consists of three parts: a sensor molecule, the adaptor protein ASC and the effector molecule pro-caspase-1. The sensors include NLRs, AIM2, and pyrin. NLRP3, 6, 7, 9, and 12 sensors all have a PYD at the N-terminus, an NBD or NACHT in the middle, and an LRR domain at the C-terminus. AIM2 consists of a PYD at the N-terminus and a HIN-200 domain at the C-terminus. ASC is required for the formation of NLRP3, 6, 7, 9, 12, and AIM2 inflammasomes. ASC mediates signaling to promote pro-caspase-1 activation through homotypic PYD-PYD or CARD-CARD interactions. The NLRP1 sensor also has a PYD at the N-terminus and a CARD at the C-terminus, which can bind directly to caspase-1 independent of ASC, and NLRP1 has a unique FIIND domain that is involved in inflammasome activation through its own protein hydrolysis. NLRC4 has an N-terminal CARD, an intermediate NBD or NACHT, and a C-terminal LRR domain. NAIP is required for NLRC4 to recognize PAMP, and then NLRC4 self-activates and oligomerizes to form the NAIP-NLRC4 inflammasome. Noncanonical inflammasomes include human caspase-4, caspase-5, and murine caspase-11. ASC, apoptosis-associated speck-like protein containing a caspase recruitment domain; AIM2, absent in melanoma AIM2; PYD, pyrin domain; NBD, nucleotide-binding domain; CARD, caspase recruitment domain; PAMP, pathogen-associated molecular patterns.