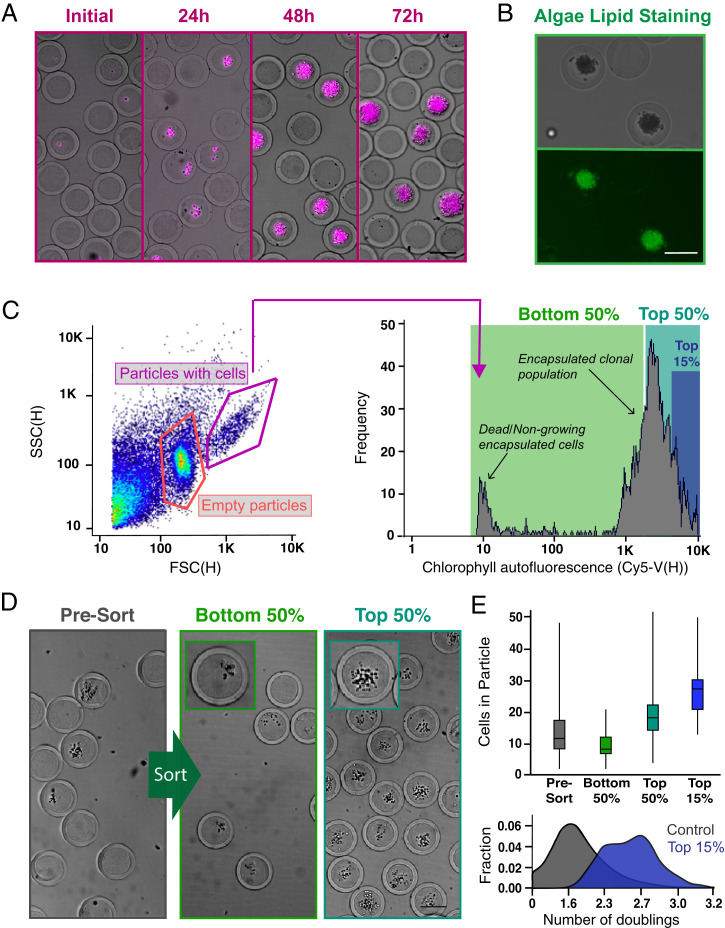
Fig. 3.
Screening and sorting characterization of microalgae-containing PicoShells. (A) PicoShells were loaded with Chlorella at lambda = 0.1 and allowed to grow for 48 h. The growth of Chlorella can be characterized via the chlorophyll autofluorescence that appears in the Cy5 channel. (B) The lipids in encapsulated Chlorella cells were stained with the addition of BODIPY 505/515. Localization of the stain was observed in the FITC channel. (C) After allowing Chlorella to divide in PicoShells, the particles were screened using an On-Chip Biotechnologies Cell Sorter. Particles that contain colonies and cells can be distinguished from empty particles using scatter readouts. Colony-containing particles produce an observable Cy5 fluorescence distribution via the colony’s chlorophyll autofluorescence. SSC(H), side scatter height. (D) The colony-containing particles that produced the lowest 50%, highest 50%, and highest 15% of Cy5 fluorescence readouts were sorted with 94.0% purity and 72.7% yield; 400 particles were sorted in each sample. Insets show magnified views of a colony within a single PicoShell for the correspondingsort gate. (E) Selection of colony-containing PicoShells from different regions of the Cy5 distribution corresponds to particles containing different numbers of algal cells, with particles with higher Cy5 fluorescence readouts containing more cells than those with lower Cy5 fluorescent readouts. Particles sorted from the higher end of the Cy5 distribution contain colonies that have undergone more doublings and have divided more during the incubation period. The middle line within each of the boxes in the box and whisker plot represents the mean number of cells in the particle; the top and bottom of each box represent the first and third quartiles, respectively; and the top and bottom of the error bars represent the maximum and minimum values, respectively. In total, 350 to 400 PicoShells were counted in each sample. (Scale bars: 50 µm.)